Efficacy and Safety of Mirvetuximab Soravtansine in Patients With Platinum-Resistant Ovarian Cancer With High Folate Receptor Alpha Expression: Results From the SORAYA Study
- PMID: 36716407
- PMCID: PMC10150846
- DOI: 10.1200/JCO.22.01900
Efficacy and Safety of Mirvetuximab Soravtansine in Patients With Platinum-Resistant Ovarian Cancer With High Folate Receptor Alpha Expression: Results From the SORAYA Study
Abstract
Purpose: Single-agent chemotherapies have limited activity and considerable toxicity in patients with platinum-resistant epithelial ovarian cancer (PROC). Mirvetuximab soravtansine (MIRV) is an antibody-drug conjugate targeting folate receptor α (FRα). SORAYA is a single-arm, phase II study evaluating efficacy and safety of MIRV in patients with PROC.
Methods: SORAYA enrolled FRα-high patients with PROC who had received one to three prior therapies, including required bevacizumab. The primary end point was confirmed objective response rate (ORR) by investigator; duration of response was the key secondary end point.
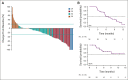
Results: One hundred six patients were enrolled; 105 were evaluable for efficacy. All patients had received prior bevacizumab, 51% had three prior lines of therapy, and 48% received a prior poly ADP-ribose polymerase inhibitor. Median follow-up was 13.4 months. ORR was 32.4% (95% CI, 23.6 to 42.2), including five complete and 29 partial responses. The median duration of response was 6.9 months (95% CI, 5.6 to 9.7). In patients with one to two priors, the ORR by investigator was 35.3% (95% CI, 22.4 to 49.9) and in patients with three priors was 30.2% (95% CI, 18.3 to 44.3). The ORR by investigator was 38.0% (95% CI, 24.7 to 52.8) in patients with prior poly ADP-ribose polymerase inhibitor exposure and 27.5% (95% CI, 15.9 to 41.7) in those without. The most common treatment-related adverse events (all grade and grade 3-4) were blurred vision (41% and 6%), keratopathy (29% and 9%), and nausea (29% and 0%). Treatment-related adverse events led to dose delays, reductions, and discontinuations in 33%, 20%, and 9% of patients, respectively.
Conclusion: MIRV demonstrated consistent clinically meaningful antitumor activity and favorable tolerability and safety in patients with FRα-high PROC who had received up to three prior therapies, including bevacizumab, representing an important advance for this biomarker-selected population.
Trial registration: ClinicalTrials.gov NCT04296890.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Armstrong DK, Alvarez RD, Backes FJ, et al. : NCCN Guidelines insights: Ovarian cancer, version 3.2022. J Natl Compr Canc Netw 20:972-980, 2022 - PubMed
-
- ten Bokkel Huinink W, Gore M, Carmichael J, et al. : Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 15:2183-2193, 1997 - PubMed
-
- Gordon AN, Fleagle JT, Guthrie D, et al. : Recurrent epithelial ovarian carcinoma: A randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19:3312-3322, 2001 - PubMed
-
- Gore M, Oza A, Rustin G, et al. : A randomised trial of oral versus intravenous topotecan in patients with relapsed epithelial ovarian cancer. Eur J Cancer 38:57-63, 2002 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

