Homologous Recombination Repair Gene Mutations to Predict Olaparib Plus Bevacizumab Efficacy in the First-Line Ovarian Cancer PAOLA-1/ENGOT-ov25 Trial
- PMID: 36716415
- PMCID: PMC9928987
- DOI: 10.1200/PO.22.00258
Homologous Recombination Repair Gene Mutations to Predict Olaparib Plus Bevacizumab Efficacy in the First-Line Ovarian Cancer PAOLA-1/ENGOT-ov25 Trial
Abstract
Purpose: The PAOLA-1/ENGOT-ov25 trial of maintenance olaparib plus bevacizumab for newly diagnosed advanced high-grade ovarian cancer demonstrated a significant progression-free survival (PFS) benefit over placebo plus bevacizumab, particularly in patients with homologous recombination deficiency (HRD)-positive tumors. We explored whether mutations in non-BRCA1 or BRCA2 homologous recombination repair (non-BRCA HRRm) genes predicted benefit from olaparib plus bevacizumab in PAOLA-1.
Methods: Eight hundred and six patients were randomly assigned (2:1). Tumors were analyzed using the Myriad MyChoice HRD Plus assay to assess non-BRCA HRRm and HRD status; HRD was based on a genomic instability score (GIS) of ≥ 42. In this exploratory analysis, PFS was assessed in patients harboring deleterious mutations using six non-BRCA HRR gene panels, three devised for this analysis and three previously published.
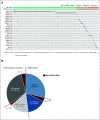
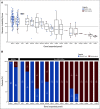
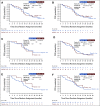
Results: The non-BRCA HRRm prevalence ranged from 30 of 806 (3.7%) to 79 of 806 (9.8%) depending on the gene panel used, whereas 152 of 806 (18.9%) had non-BRCA1 or BRCA2 mutation HRD-positive tumors. The majority of tumors harboring non-BRCA HRRm had a low median GIS; however, a GIS of > 42 was observed for tumors with mutations in five HRR genes (BLM, BRIP1, RAD51C, PALB2, and RAD51D). Rates of gene-specific biallelic loss were variable (0% to 100%) in non-BRCA HRRm tumors relative to BRCA1-mutated (99%) or BRCA2-mutated (86%) tumors. Across all gene panels tested, hazard ratios for PFS (95% CI) ranged from 0.92 (0.51 to 1.73) to 1.83 (0.76 to 5.43).
Conclusion: Acknowledging limitations of small subgroup sizes, non-BRCA HRRm gene panels were not predictive of PFS benefit with maintenance olaparib plus bevacizumab versus placebo plus bevacizumab in PAOLA-1, irrespective of the gene panel tested. Current gene panels exploring HRRm should not be considered a substitute for HRD determined by BRCA mutation status and genomic instability testing in first-line high-grade ovarian cancer.
Trial registration: ClinicalTrials.gov NCT02477644.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Ledermann JA, Raja FA, Fotopoulou C, et al. : Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:vi24-vi32, 2013. (suppl 6) - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 - PubMed
-
- O'Connor MJ: Targeting the DNA damage response in cancer. Mol Cell 60:547-560, 2015 - PubMed
-
- Ray-Coquard I, Pautier P, Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 - PubMed
-
- González-Martín A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

