Normal muscle fiber type distribution is recapitulated in aged ephrin-A3-/- mice that previously lacked most slow myofibers
- PMID: 36717102
- PMCID: PMC10027087
- DOI: 10.1152/ajpcell.00519.2022
Normal muscle fiber type distribution is recapitulated in aged ephrin-A3-/- mice that previously lacked most slow myofibers
Abstract
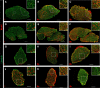
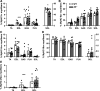
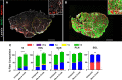
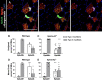
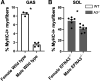
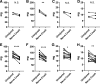
Individual limb muscles have characteristic representation and spatial distribution of muscle fiber types (one slow and up to three fast isoforms) appropriate to their unique anatomical location and function. This distribution can be altered by physiological stimuli such as training (i.e., for increased endurance or force) or pathological conditions such as aging. Our group previously showed that ephrin-A3 is expressed only on slow myofibers, and that adult mice lacking ephrin-A3 have dramatically reduced numbers of slow myofibers due to postnatal innervation of previously slow myofibers by fast motor neurons. In this study, fiber type composition of hindlimb muscles of aged and denervated/reinnervated C57BL/6 and ephrin-A3-/- mice was analyzed to determine whether the loss of slow myofibers persists across the lifespan. Surprisingly, fiber-type composition of ephrin-A3-/- mouse muscles at two years of age was nearly indistinguishable from age-matched C57BL/6 mice. After challenge with nerve crush, the percentage of IIa and I/IIa hybrid myofibers increased significantly in aged ephrin-A3-/- mice. While EphA8, the receptor for ephrin-A3, is present at all neuromuscular junctions (NMJs) on fast fibers in 3-6 mo old C57BL/6 and ephrin-A3-/- mice, this exclusive localization is lost with aging, with EphA8 expression now found on a subset of NMJs on some slow muscle fibers. This return to appropriate fiber-type distribution given time and under use reinforces the role of activity in determining fiber-type representation and suggests that, rather than being a passive baseline, the developmentally and evolutionarily selected fiber type pattern may instead be actively reinforced by daily living.
Keywords: Eph/ephrin; aging; neuromuscular junction; skeletal muscle fiber type.
Conflict of interest statement
C. L. Lorson is the co-founder and chief scientific officer for Shift Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

