Urinary markers differentially associate with kidney inflammatory activity and chronicity measures in patients with lupus nephritis
- PMID: 36717181
- PMCID: PMC9887703
- DOI: 10.1136/lupus-2022-000747
Urinary markers differentially associate with kidney inflammatory activity and chronicity measures in patients with lupus nephritis
Abstract
Objective: Lupus nephritis (LN) is diagnosed by biopsy, but longitudinal monitoring assessment methods are needed. Here, in this preliminary and hypothesis-generating study, we evaluate the potential for using urine proteomics as a non-invasive method to monitor disease activity and damage. Urinary biomarkers were identified and used to develop two novel algorithms that were used to predict LN activity and chronicity.
Methods: Baseline urine samples were collected for four cohorts (healthy donors (HDs, n=18), LN (n=42), SLE (n=17) or non-LN kidney disease biopsy control (n=9)), and over 1 year for patients with LN (n=42). Baseline kidney biopsies were available for the LN (n=46) and biopsy control groups (n=9). High-throughput proteomics platforms were used to identify urinary analytes ≥1.5 SD from HD means, which were subjected to stepwise, univariate and multivariate logistic regression modelling to develop predictive algorithms for National Institutes of Health Activity Index (NIH-AI)/National Institutes of Health Chronicity Index (NIH-CI) scores. Kidney biopsies were analysed for macrophage and neutrophil markers using immunohistochemistry (IHC).
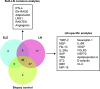
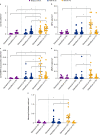
Results: In total, 112 urine analytes were identified from LN, SLE and biopsy control patients as both quantifiable and overexpressed compared with HDs. Regression analysis identified proteins associated with the NIH-AI (n=30) and NIH-CI (n=26), with four analytes common to both groups, demonstrating a difference in the mechanisms associated with NIH-AI and NIH-CI. Pathway analysis of the NIH-AI and NIH-CI analytes identified granulocyte-associated and macrophage-associated pathways, and the presence of these cells was confirmed by IHC in kidney biopsies. Four markers each for the NIH-AI and NIH-CI were identified and used in the predictive algorithms. The NIH-AI algorithm sensitivity and specificity were both 93% with a false-positive rate (FPR) of 7%. The NIH-CI algorithm sensitivity was 88%, specificity 96% and FPR 4%. The accuracy for both models was 93%.
Conclusions: Longitudinal predictions suggested that patients with baseline NIH-AI scores of ≥8 were most sensitive to improvement over 6-12 months. Viable approaches such as this may enable the use of urine samples to monitor LN over time.
Keywords: inflammation; lupus erythematosus, systemic; lupus nephritis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AA, LZ and JAC were formerly employed by, and may hold stock in, AstraZeneca. DS, DC and WIW are currently employed by, and own stock in, AstraZeneca. ABF, JC, MB and SSL’s institution has a sponsored research agreement with AstraZeneca. JAC has participated on an AstraZeneca advisory board. GGI was formerly employed by, and may hold stock in, Horizon Therapeutics. Support for the present study and manuscript editorial assistance were provided by AstraZeneca and Fishawack Health.
Figures


References
-
- El-Gendi S, Abdul-Hamid S, Ahmed A, et al. Urinary kidney injury molecule 1 (U-KIM-1) as a predictor of lupus nephritis. Medical Journal of Cairo University 2018;86:2621–31.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
