Biology of the SARS-CoV-2 Coronavirus
- PMID: 36717455
- PMCID: PMC9839213
- DOI: 10.1134/S0006297922120215
Biology of the SARS-CoV-2 Coronavirus
Abstract
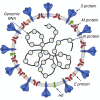
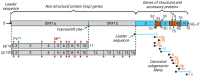
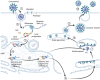
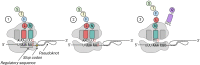
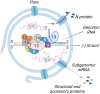
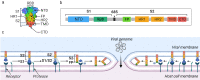
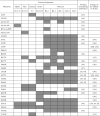
New coronavirus infection causing COVID-19, which was first reported in late 2019 in China, initiated severe social and economic crisis that affected the whole world. High frequency of the errors in replication of RNA viruses, zoonotic nature of transmission, and high transmissibility allowed betacoronaviruses to cause the third pandemic in the world since the beginning of 2003: SARS-CoV in 2003, MERS-CoV in 2012, and SARS-CoV-2 in 2019. The latest pandemic united scientific community and served as a powerful impetus in the study of biology of coronaviruses: new routes of virus penetration into the human cells were identified, features of the replication cycle were studied, and new functions of coronavirus proteins were elucidated. It should be recognized that the pandemic was accompanied by the need to obtain and publish results within a short time, which led to the emergence of an array of conflicting data and low reproducibility of research results. We systematized and analyzed scientific literature, filtered the results according to reliability of the methods of analysis used, and prepared a review describing molecular mechanisms of functioning of the SARS-CoV-2 coronavirus. This review considers organization of the genome of the SARS-CoV-2 virus, mechanisms of its gene expression and entry of the virus into the cell, provides information on key mutations that characterize different variants of the virus, and their contribution to pathogenesis of the disease.
Keywords: COVID-19; S protein; SARS-CoV-2; VOC; mutation.
Conflict of interest statement
The authors declare no conflicts of interests in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Figures

Similar articles
-
Molecular Basis of Pathogenesis of Coronaviruses: A Comparative Genomics Approach to Planetary Health to Prevent Zoonotic Outbreaks in the 21st Century.OMICS. 2020 Nov;24(11):634-644. doi: 10.1089/omi.2020.0131. Epub 2020 Sep 16. OMICS. 2020. PMID: 32940573 Review.
-
Emerging coronaviruses: first SARS, second MERS and third SARS-CoV-2: epidemiological updates of COVID-19.Infez Med. 2020 Jun 1;28(suppl 1):6-17. Infez Med. 2020. PMID: 32532933 Review.
-
Human and novel coronavirus infections in children: a review.Paediatr Int Child Health. 2021 Feb;41(1):36-55. doi: 10.1080/20469047.2020.1781356. Epub 2020 Jun 25. Paediatr Int Child Health. 2021. PMID: 32584199 Review.
-
Amplicon and Metagenomic Analysis of Middle East Respiratory Syndrome (MERS) Coronavirus and the Microbiome in Patients with Severe MERS.mSphere. 2021 Aug 25;6(4):e0021921. doi: 10.1128/mSphere.00219-21. Epub 2021 Jul 21. mSphere. 2021. PMID: 34287009 Free PMC article.
-
Rampant C→U Hypermutation in the Genomes of SARS-CoV-2 and Other Coronaviruses: Causes and Consequences for Their Short- and Long-Term Evolutionary Trajectories.mSphere. 2020 Jun 24;5(3):e00408-20. doi: 10.1128/mSphere.00408-20. mSphere. 2020. PMID: 32581081 Free PMC article.
Cited by
-
Detection of SARS-CoV-2 Based on Nucleic Acid Amplification Tests (NAATs) and Its Integration into Nanomedicine and Microfluidic Devices as Point-of-Care Testing (POCT).Int J Mol Sci. 2023 Jun 16;24(12):10233. doi: 10.3390/ijms241210233. Int J Mol Sci. 2023. PMID: 37373381 Free PMC article. Review.
-
Molecular Insights into Structural Dynamics and Binding Interactions of Selected Inhibitors Targeting SARS-CoV-2 Main Protease.Int J Mol Sci. 2024 Dec 16;25(24):13482. doi: 10.3390/ijms252413482. Int J Mol Sci. 2024. PMID: 39769245 Free PMC article.
-
Obesity as a Risk Factor for the Severity of COVID-19 in Pediatric Patients: Possible Mechanisms-A Narrative Review.Children (Basel). 2024 Sep 30;11(10):1203. doi: 10.3390/children11101203. Children (Basel). 2024. PMID: 39457167 Free PMC article. Review.
-
Strategy for the Construction of SARS-CoV-2 S and N Recombinant Proteins and Their Immunogenicity Evaluation.BioTech (Basel). 2025 May 23;14(2):38. doi: 10.3390/biotech14020038. BioTech (Basel). 2025. PMID: 40558387 Free PMC article.
-
Diagnostic Usefulness of Serum Hyaluronic Acid in Patients with SARS-CoV-2 Infection.J Clin Med. 2024 Dec 8;13(23):7471. doi: 10.3390/jcm13237471. J Clin Med. 2024. PMID: 39685929 Free PMC article.
References
-
- Reagan R. L., Yancey F., Brueckner A. L. Studies of the avian infectious bronchitis virus (wachtel strain) in the cynomolgus monkey. Poultry Sci. 1955;34:1448. doi: 10.3382/ps.0341448. - DOI
-
- Paul D., Kolar P., Hall S. G. A review of the impact of environmental factors on the fate and transport of coronaviruses in aqueous environments. npj Clean Water. 2021;4:7. doi: 10.1038/s41545-020-00096-w. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

