The interaction of β-arrestin1 with talin1 driven by endothelin A receptor as a feature of α5β1 integrin activation in high-grade serous ovarian cancer
- PMID: 36717550
- PMCID: PMC9886921
- DOI: 10.1038/s41419-023-05612-7
The interaction of β-arrestin1 with talin1 driven by endothelin A receptor as a feature of α5β1 integrin activation in high-grade serous ovarian cancer
Erratum in
-
Author Correction: The interaction of β-arrestin1 with talin1 driven by endothelin A receptor as a feature of α5β1 integrin activation in high-grade serous ovarian cancer.Cell Death Dis. 2024 Oct 21;15(10):766. doi: 10.1038/s41419-024-07139-x. Cell Death Dis. 2024. PMID: 39433739 Free PMC article. No abstract available.
Abstract
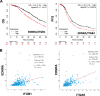
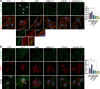
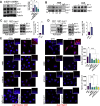
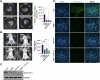
Dissemination of high-grade serous ovarian cancer (HG-SOC) in the omentum and intercalation into a mesothelial cell (MC) monolayer depends on functional α5β1 integrin (Intα5β1) activity. Although the binding of Intα5β1 to fibronectin drives these processes, other molecular mechanisms linked to integrin inside-out signaling might support metastatic dissemination. Here, we report a novel interactive signaling that contributes to Intα5β1 activation and accelerates tumor cells toward invasive disease, involving the protein β-arrestin1 (β-arr1) and the activation of the endothelin A receptor (ETAR) by endothelin-1 (ET-1). As demonstrated in primary HG-SOC cells and SOC cell lines, ET-1 increased Intβ1 and downstream FAK/paxillin activation. Mechanistically, β-arr1 directly interacts with talin1 and Intβ1, promoting talin1 phosphorylation and its recruitment to Intβ1, thus fueling integrin inside-out activation. In 3D spheroids and organotypic models mimicking the omentum, ETAR/β-arr1-driven Intα5β1 signaling promotes the survival of cell clusters, with mesothelium-intercalation capacity and invasive behavior. The treatment with the antagonist of ETAR, Ambrisentan (AMB), and of Intα5β1, ATN161, inhibits ET-1-driven Intα5β1 activity in vitro, and tumor cell adhesion and spreading to intraperitoneal organs and Intβ1 activity in vivo. As a prognostic factor, high EDNRA/ITGB1 expression correlates with poor HG-SOC clinical outcomes. These findings highlight a new role of ETAR/β-arr1 operating an inside-out integrin activation to modulate the metastatic process and suggest that in the new integrin-targeting programs might be considered that ETAR/β-arr1 regulates Intα5β1 functional pathway.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

