Progenitor-derived endothelin controls dermal sheath contraction for hair follicle regression
- PMID: 36717629
- PMCID: PMC9931655
- DOI: 10.1038/s41556-022-01065-w
Progenitor-derived endothelin controls dermal sheath contraction for hair follicle regression
Abstract
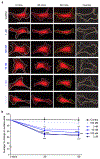
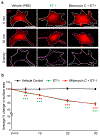
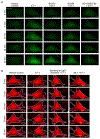
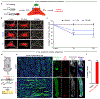
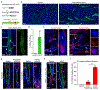
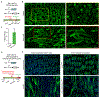
Substantial follicle remodelling during the regression phase of the hair growth cycle is coordinated by the contraction of the dermal sheath smooth muscle, but how dermal-sheath-generated forces are regulated is unclear. Here, we identify spatiotemporally controlled endothelin signalling-a potent vasoconstriction-regulating pathway-as the key activating mechanism of dermal sheath contraction. Pharmacological blocking or genetic ablation of both endothelin receptors, ETA and ETB, impedes dermal sheath contraction and halts follicle regression. Epithelial progenitors at the club hair-epithelial strand bottleneck produce the endothelin ligand ET-1, which is required for follicle regression. ET signalling in dermal sheath cells and downstream contraction is dynamically regulated by cytoplasmic Ca2+ levels through cell membrane and sarcoplasmic reticulum calcium channels. Together, these findings illuminate an epithelial-mesenchymal interaction paradigm in which progenitors-destined to undergo programmed cell death-control the contraction of the surrounding sheath smooth muscle to orchestrate homeostatic tissue regression and reorganization for the next stem cell activation and regeneration cycle.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures











Comment in
-
Endothelin signalling drives dermal sheath smooth muscle contraction for hair follicle regression.Nat Cell Biol. 2023 Feb;25(2):220-221. doi: 10.1038/s41556-022-01077-6. Nat Cell Biol. 2023. PMID: 36717628 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

