Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL
- PMID: 36717654
- PMCID: PMC9991909
- DOI: 10.1038/s41375-023-01829-9
Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL
Abstract
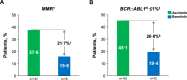
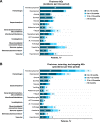
Asciminib, the first BCR::ABL1 inhibitor that Specifically Targets the ABL Myristoyl Pocket (STAMP), is approved worldwide for the treatment of adults with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (CML-CP) treated with ≥2 prior tyrosine kinase inhibitors (TKIs). In ASCEMBL, patients with CML-CP treated with ≥2 prior TKIs were randomized (stratified by baseline major cytogenetic response [MCyR]) 2:1 to asciminib 40 mg twice daily or bosutinib 500 mg once daily. Consistent with previously published primary analysis results, after a median follow-up of 2.3 years, asciminib continued to demonstrate superior efficacy and better safety and tolerability than bosutinib. The major molecular response (MMR) rate at week 96 (key secondary endpoint) was 37.6% with asciminib vs 15.8% with bosutinib; the MMR rate difference between the arms, after adjusting for baseline MCyR, was 21.7% (95% CI, 10.53-32.95; two-sided p = 0.001). Fewer grade ≥3 adverse events (AEs) (56.4% vs 68.4%) and AEs leading to treatment discontinuation (7.7% vs 26.3%) occurred with asciminib than with bosutinib. A higher proportion of patients on asciminib than bosutinib remained on treatment and continued to derive benefit over time, supporting asciminib as a standard of care for patients with CML-CP previously treated with ≥2 TKIs.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following potential conflicts of interest: AH received institutional research support from Novartis, Bristol Myers Squibb, Incyte, and Pfizer and personal fees from Novartis and Incyte. DR received personal fees from Novartis, Pfizer, and Incyte. CB received grants from Novartis during the conduct of the study. YM received honoraria from Bristol Myers Squibb, Novartis, Pfizer, and Astellas. JEC received grants and consulting fees from Novartis, grants and consulting fees from Pfizer, and grants from Bristol Myers Squibb. TPH received grants and honoraria for advisory boards and symposia from Novartis and grants from Bristol Myers Squibb. JFA received honoraria, grants, and personal fees from Novartis and grants and personal fees from Pfizer. EL received grants from Novartis and personal fees and non-financial support from Novartis, Pfizer, and Bristol Myers Squibb. SV received personal fees and non-financial support from Novartis, AbbVie, Janssen, Sanofi, and BIOCAD; non-financial support from Pfizer; and personal fees from Takeda and AstraZeneca. AT declares no competing financial interests. D-WK received grants from Novartis, Bristol Myers Squibb, Pfizer, ILYANG, and Takeda. AAbdo received honoraria from Novartis and Takeda. LMF declares no competing financial interests. PlC received speaker’s honoraria from Novartis, Incyte, Pfizer, and Bristol Myers Squibb. KS received research funding and honoraria for advisory boards from Novartis. DDHK received grants, honoraria, and consulting fees from Novartis; grants from Bristol Myers Squibb; and honoraria from Pfizer and Paladin. SS received honoraria, grants, and personal fees from Novartis and grants and personal fees from Bristol Myers Squibb and Incyte, and honoraria from Roche and Pfizer. MA declares no competing financial interests. NC declares no competing financial interests. LC received honoraria and participated in advisory boards for Novartis and Otsuka. VG-G received grants, non-financial support, and honoraria from Novartis, Pfizer, Bristol Myers Squibb, and Incyte. SK is an employee of Novartis. AAllepuz is an employee of Novartis. SQ is an employee of Novartis. VB is an employee of Novartis. MJM received personal fees from Bristol Myers Squibb, Takeda, and Pfizer.
Figures


References
-
- Chronic Myeloid Leukemia, V1.2023, NCCN Clinical Practice Guidelines in Oncology.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

