Modeling of intracranial tumor treating fields for the treatment of complex high-grade gliomas
- PMID: 36717682
- PMCID: PMC9886948
- DOI: 10.1038/s41598-023-28769-9
Modeling of intracranial tumor treating fields for the treatment of complex high-grade gliomas
Erratum in
-
Author Correction: Modeling of intracranial tumor treating fields for the treatment of complex high-grade gliomas.Sci Rep. 2023 Mar 9;13(1):3955. doi: 10.1038/s41598-023-30970-9. Sci Rep. 2023. PMID: 36894660 Free PMC article. No abstract available.
Abstract
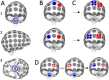
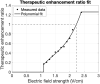
Increasing the intensity of tumor treating fields (TTF) within a tumor bed improves clinical efficacy, but reaching sufficiently high field intensities to achieve growth arrest remains challenging due in part to the insulating nature of the cranium. Using MRI-derived finite element models (FEMs) and simulations, we optimized an exhaustive set of intracranial electrode locations to obtain maximum TTF intensities in three clinically challenging high-grade glioma (HGG) cases (i.e., thalamic, left temporal, brainstem). Electric field strengths were converted into therapeutic enhancement ratios (TER) to evaluate the predicted impact of stimulation on tumor growth. Concurrently, conventional transcranial configurations were simulated/optimized for comparison. Optimized intracranial TTF were able to achieve field strengths that have previously been shown capable of inducing complete growth arrest, in 98-100% of the tumor volumes using only 0.54-0.64 A current. The reconceptualization of TTF as a targeted, intracranial therapy has the potential to provide a meaningful survival benefit to patients with HGG and other brain tumors, including those in surgically challenging, deep, or anatomically eloquent locations which may preclude surgical resection. Accordingly, such an approach may ultimately represent a paradigm shift in the use of TTFs for the treatment of brain cancer.
© 2023. The Author(s).
Conflict of interest statement
DJS is an officer and board member with equity in Saturn5 Corporation. JDB has an equity position in Treovir LLC and is a member of the POCKiT Diagnostics Board of Scientific Advisors. GKF is supported by Eli Lilly and Company and Pfizer through contracts to UAB. The remaining authors declare no pertinent conflicts of interest.
Figures




References
-
- Stupp R, Kanner A, Engelhard H, et al. A prospective, randomized, open-label, phase III clinical trial of NovoTTF-100A versus best standard of care chemotherapy in patients with recurrent glioblastoma. J. Clin. Oncol. 2010;28(18 suppl):LBA2007. doi: 10.1200/jco.2010.28.18_suppl.lba2007. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

