Adverse perinatal outcomes associated with prenatal exposure to protease-inhibitor-based versus non-nucleoside reverse transcriptase inhibitor-based antiretroviral combinations in pregnant women with HIV infection: a systematic review and meta-analysis
- PMID: 36717801
- PMCID: PMC9885641
- DOI: 10.1186/s12884-023-05347-5
Adverse perinatal outcomes associated with prenatal exposure to protease-inhibitor-based versus non-nucleoside reverse transcriptase inhibitor-based antiretroviral combinations in pregnant women with HIV infection: a systematic review and meta-analysis
Abstract
Background: About 1.3 million pregnant women lived with HIV and were eligible to receive antiretroviral therapy (ART) worldwide in 2021. The World Health Organization recommends protease inhibitors (PI)-based regimen as second or third-line during pregnancy. With remaining pregnant women exposed to PIs, there is still an interest to assess whether this treatment affects perinatal outcomes. Adverse perinatal outcomes after prenatal exposure to PI-based ART remain conflicting: some studies report an increased risk of preterm birth (PTB) and low-birth-weight (LBW), while others do not find these results. We assessed adverse perinatal outcomes associated with prenatal exposure to PI-based compared with non-nucleoside reverse transcriptase (NNRTI)-based ART.
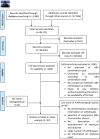
Methods: We performed a systematic review searching PubMed, Reprotox, Clinical Trial Registry (clinicaltrials.gov) and abstracts of HIV conferences between 01/01/2002 and 29/10/2021. We used Oxford and Newcastle-Ottawa scales to assess the methodological quality. Studied perinatal outcomes were spontaneous abortion, stillbirth, congenital abnormalities, PTB (< 37 weeks of gestation), very preterm birth (VPTB, < 32 weeks of gestation), LBW (< 2500 grs), very low-birth-weight (VLBW, < 1500 g), small for gestational age (SGA) and very small for gestational age (VSGA). The association between prenatal exposure to PI-based compared to NNRTI-based ART was measured for each adverse perinatal outcome using random-effect meta-analysis to estimate pooled relative risks (RR) and their corresponding 95% confidence intervals (CI). Pre-specified analyses were stratified according to country income and study quality assessment, and summarized when homogeneous.
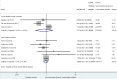
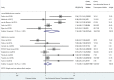
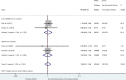
Results: Out of the 49,171 citations identified, our systematic review included 32 published studies, assessing 45,427 pregnant women. There was no significant association between prenatal exposure to PIs compared to NNRTIs for VPTB, LBW, SGA, stillbirth, and congenital abnormalities. However, it was inconclusive for PTB, and PI-based ART is significantly associated with an increased risk of VSGA (sRR 1.41 [1.08-1.84]; I2 = 0%) compared to NNRTIs.
Conclusions: We did not report any significant association between prenatal exposure to PIs vs NNRTIs-based regimens for most of the adverse perinatal outcomes, except for VSGA significantly increased (+ 41%). The evaluation of antiretroviral exposure on pregnancy outcomes remains crucial to fully assess the benefice-risk balance, when prescribing ART in women of reproductive potential with HIV.
Prospero number: CRD42022306896.
Keywords: Antiretroviral therapy; HIV; Perinatal outcomes; Pregnancy; Protease inhibitor; Systematic review.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
-
- UNAIDS. Global HIV & AIDS statistics — Fact sheet 2022 n.d. https://www.unaids.org/en/resources/fact-sheet (accessed August 2, 2022).
-
- WHO | Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: what’s new. WHO n.d. http://www.who.int/hiv/pub/arv/policy-brief-arv-2015/en/ (accessed January 16, 2019).
-
- WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV guidelines 2015. http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?... (accessed June 10, 2018). - PubMed
-
- WHO. HIV/AIDS - Key facts 2020. https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed January 9, 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

