Establishment and characterization of a HER2-enriched canine mammary cancerous myoepithelial cell line
- PMID: 36717813
- PMCID: PMC9885638
- DOI: 10.1186/s12917-023-03573-9
Establishment and characterization of a HER2-enriched canine mammary cancerous myoepithelial cell line
Abstract
Background: Canine mammary tumors (CMTs) have a poor prognosis, along with tumor recurrence and metastasis. Cell lines are vital in vitro models for CMT research. Many CMT epithelial cell lines were reported. However, canine mammary myoepithelial cells, the contractile component of the canine mammary tissue were overlooked. This study aimed at establishing such a cell line. CMT-1 cell line was obtained from a canine mammary tumor CMT-1 and characterized molecularly through qPCR, western blotting, immunochemistry and immunofluorescence. Its doubling time, cytogenetic analysis and migration rate were evaluated using growth study, karyotype analysis and wound healing assay respectively. To determine its tumorigenesis, xenograft transplantation was performed.
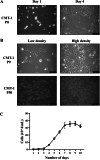
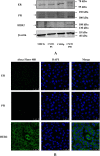
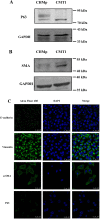
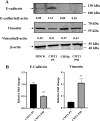
Results: CMT-1 tumor was a complex canine mammary carcinoma that stained negative to estrogen receptors (ER) and progesterone receptors (PR), but positive to human epidermal growth receptor-2 (HER2), defined as HER2-enriched subtype. In this study, a CMT-1 cell line obtained from CMT-1 tumor was immune-positive to vimentin, α-SMA, p63 and negative to E-cadherin (E-cad), indicating CMT-1 cells were myoepithelial cells. It was successfully cultured for more than 50 passages showing the same immunoreactivity to ER, PR, and HER2 as the primary canine tumor. The doubling time of CMT-1 cell line was 26.67 h. The chromosome number of CMT-1 cells ranged from 31 to 64. A potential spontaneous epithelial to mesenchymal transition (EMT) was noticed during cell cultures. Potential EMT-induced CMT-1 cells showed no significance in migration rate compared to the original CMT-1 cells. CMT-1 cells was able to grow on a 3D culture and formed grape-like, solid, and cystic mammospheres at different time period. Inoculation of CMT-1 cells induced a complex HER2-enriched mammary tumor with metastasis in mice.
Conclusions: A canine cancerous HER2-enriched myoepithelial cell line was successfully established and a canine mammosphere developed from myoepithelial cells was documented in this study. We are expecting this novel cell line and its associated mammospheres could be used as a model to elucidate the role of myoepithelial cells in CMT carcinogensis in the future.
Keywords: CMT; EMT; HER2; Mammosphere; Myoepithelium.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Misdorp WER, Hellmen E, Lipscomb TP. Histologic Classification of Mammary Tumors of the Dog and the Cat, 2nd ser. Washington, DC: Armed Force Institute of Pathology and World Health Organization; 1999.
-
- Pena L, Gama A, Goldschmidt MH, Abadie J, Benazzi C, Castagnaro M, Diez L, Gartner F, Hellmen E, Kiupel M, et al. Canine mammary tumors: a review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet Pathol. 2014;51(1):127–145. doi: 10.1177/0300985813509388. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

