A therapeutic target for CKD: activin A facilitates TGFβ1 profibrotic signaling
- PMID: 36717814
- PMCID: PMC9885651
- DOI: 10.1186/s11658-023-00424-1
A therapeutic target for CKD: activin A facilitates TGFβ1 profibrotic signaling
Abstract
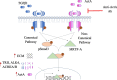
Background: TGFβ1 is a major profibrotic mediator in chronic kidney disease (CKD). Its direct inhibition, however, is limited by adverse effects. Inhibition of activins, also members of the TGFβ superfamily, blocks TGFβ1 profibrotic effects, but the mechanism underlying this and the specific activin(s) involved are unknown.
Methods: Cells were treated with TGFβ1 or activins A/B. Activins were inhibited generally with follistatin, or specifically with neutralizing antibodies or type I receptor downregulation. Cytokine levels, signaling and profibrotic responses were assessed with ELISA, immunofluorescence, immunoblotting and promoter luciferase reporters. Wild-type or TGFβ1-overexpressing mice with unilateral ureteral obstruction (UUO) were treated with an activin A neutralizing antibody.
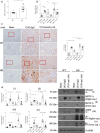
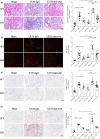
Results: In primary mesangial cells, TGFβ1 induces secretion primarily of activin A, which enables longer-term profibrotic effects by enhancing Smad3 phosphorylation and transcriptional activity. This results from lack of cell refractoriness to activin A, unlike that for TGFβ1, and promotion of TGFβ type II receptor expression. Activin A also supports transcription through regulating non-canonical MRTF-A activation. TGFβ1 additionally induces secretion of activin A, but not B, from tubular cells, and activin A neutralization prevents the TGFβ1 profibrotic response in renal fibroblasts. Fibrosis induced by UUO is inhibited by activin A neutralization in wild-type mice. Worsened fibrosis in TGFβ1-overexpressing mice is associated with increased renal activin A expression and is inhibited to wild-type levels with activin A neutralization.
Conclusions: Activin A facilitates TGFβ1 profibrotic effects through regulation of both canonical (Smad3) and non-canonical (MRTF-A) signaling, suggesting it may be a novel therapeutic target for preventing fibrosis in CKD.
Keywords: Activin A; Extracellular matrix; Kidney fibrosis; TGFβ1.
© 2023. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures





References
-
- Moody WE, Chue CD, Inston NG, Edwards NC, Steeds RP, Ferro CJ, et al. Understanding the effects of chronic kidney disease on cardiovascular risk: are there lessons to be learnt from healthy kidney donors? J Hum Hypertens. 2012;26(3):141–148. - PubMed
-
- Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. - PubMed
-
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–338. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

