Characterization of large extracellular vesicles (L-EV) derived from human regulatory macrophages (Mreg): novel mediators in wound healing and angiogenesis?
- PMID: 36717876
- PMCID: PMC9887800
- DOI: 10.1186/s12967-023-03900-6
Characterization of large extracellular vesicles (L-EV) derived from human regulatory macrophages (Mreg): novel mediators in wound healing and angiogenesis?
Abstract
Background: Large extracellular vesicles (L-EV) with a diameter between 1 and 10 µm are released by various cell types. L-EV contain and transport active molecules which are crucially involved in cell to cell communication. We have shown that secretory products of human regulatory macrophages (Mreg) bear pro-angiogenic potential in-vitro and our recent findings show that Mreg cultures also contain numerous large vesicular structures similar to L-EV with so far unknown characteristics and function.
Aim of this study: To characterize the nature of Mreg-derived L-EV (L-EVMreg) and to gain insights into their role in wound healing and angiogenesis.
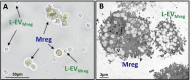
Methods: Mreg were differentiated using blood monocytes from healthy donors (N = 9) and L-EVMreg were isolated from culture supernatants by differential centrifugation. Characterization of L-EVMreg was performed by cell/vesicle analysis, brightfield/transmission electron microscopy (TEM), flow cytometry and proteome profiling arrays. The impact of L-EVMreg on wound healing and angiogenesis was evaluated by means of scratch and in-vitro tube formation assays.
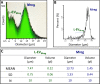
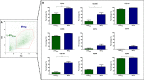
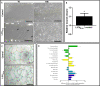
Results: Mreg and L-EVMreg show an average diameter of 13.73 ± 1.33 µm (volume: 1.45 ± 0.44 pl) and 7.47 ± 0.75 µm (volume: 0.22 ± 0.06 pl) respectively. Flow cytometry analyses revealed similarities between Mreg and L-EVMreg regarding their surface marker composition. However, compared to Mreg fewer L-EVMreg were positive for CD31 (P < 0.01), CD206 (P < 0.05), CD103 (P < 0.01) and CD45 (P < 0.05). Proteome profiling suggested that L-EVMreg contain abundant amounts of pro-angiogenic proteins (i.e. interleukin-8, platelet factor 4 and serpin E1). From a functional point of view L-EVMreg positively influenced in-vitro wound healing (P < 0.05) and several pro-angiogenic parameters in tube formation assays (all segment associated parameters, P < 0.05; number of meshes, P < 0.05).
Conclusion: L-EVMreg with regenerative and pro-angiogenic potential can be reproducibly isolated from in-vitro cultured human regulatory macrophages. We propose that L-EVMreg could represent a putative therapeutic option for the treatment of chronic wounds and ischemia-associated diseases.
Keywords: Angiogenesis; Large extracellular vesicles; Macrophages; Wound healing.
© 2023. The Author(s).
Conflict of interest statement
The authors (MA, KZ, FF, RB) are involved in a pending patent concerning L-EVMreg and results of the current study are included in the patent application.
Figures


References
-
- Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 2019;405:148–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

