Avelumab in Combination With Cetuximab and Chemotherapy as First-Line Treatment for Patients With Advanced Squamous NSCLC
- PMID: 36718142
- PMCID: PMC9883276
- DOI: 10.1016/j.jtocrr.2022.100461
Avelumab in Combination With Cetuximab and Chemotherapy as First-Line Treatment for Patients With Advanced Squamous NSCLC
Abstract
Introduction: We present the results of a phase 2a trial of first-line avelumab (anti-programmed death-ligand 1 antibody) plus cetuximab (anti-EGFR antibody) in patients with advanced squamous NSCLC.
Methods: Patients with recurrent or metastatic squamous NSCLC received avelumab 800 mg (d 1 and 8), cetuximab 250 mg/m2 (d 1) and 500 mg/m2 (d 8), cisplatin 75 mg/m2 (d 1), and gemcitabine 1250 mg/m2 (d 1 and 8) for four 3-week cycles, followed by avelumab 800 mg and cetuximab 500 mg/m2 every 2 weeks. The primary end point was the best overall response; the secondary end points were progression-free survival, duration of response, overall survival, and safety. Efficacy analyses were reported from an updated data cutoff.
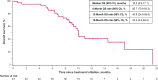
Results: A total of 43 patients were enrolled. The median follow-up was 6.6 months for the primary analyses and 9.2 months for the efficacy analyses. In the efficacy analyses, 15 patients had a confirmed partial response (objective response rate, 34.9% [95% confidence interval: 21.0%-50.9%]), and the median duration of response was 7.1 months (95% confidence interval: 4.2-12.5 mo). The median progression-free survival and overall survival were 6.1 months and 10.0 months, respectively. In the safety analyses (primary analysis), 38 patients (88.4%) had a treatment-related adverse event, of whom 24 (55.8%) had a grade 3 or higher treatment-related adverse event.
Conclusions: The combination of avelumab + cetuximab and chemotherapy showed antitumor activity and tolerable safety; however, the ORR was not improved compared with those reported for current standards of care (NCT03717155).
Keywords: Avelumab; Cetuximab; EGFR; Non–small cell lung cancer; PD-L1.
© 2023 Published by Elsevier Inc. on behalf of the International Association for the Study of Lung Cancer.
Figures


References
-
- NCCN Clinical Practice Guidelines in Oncology . V5; 2022. Non-small cell lung cancer.https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
-
- European Society for Medical Oncology Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice... - PubMed
-
- Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. - PubMed
-
- Spigel D., de Marinis F., Giaccone G., et al. LBA78 - IMpower110: interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann Oncol. 2019;30(suppl 5):v915.
-
- Sezer A., Kilickap S., Gümüş M., et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592–604. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

