Photoinduced Electron Transfer from the Tryptophan Triplet State in Zn-Azurin
- PMID: 36718260
- PMCID: PMC9881450
- DOI: 10.1021/acsphyschemau.2c00042
Photoinduced Electron Transfer from the Tryptophan Triplet State in Zn-Azurin
Abstract
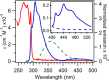
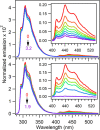
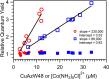
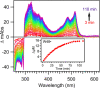
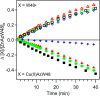
Tryptophan is one of few residues that participates in biological electron transfer reactions. Upon substitution of the native Cu2+ center with Zn2+ in the blue-copper protein azurin, a long-lived tryptophan neutral radical can be photogenerated. We report the following quantum yield values for Zn-substituted azurin in the presence of the electron acceptor Cu(II)-azurin: formation of the tryptophan neutral radical (Φrad), electron transfer (ΦET), fluorescence (Φfluo), and phosphorescence (Φphos), as well as the efficiency of proton transfer of the cation radical (ΦPT). Increasing the concentration of the electron acceptor increased Φrad and ΦET values and decreased Φphos without affecting Φfluo. At all concentrations of the acceptor, the value of ΦPT was nearly unity. These observations indicate that the phosphorescent triplet state is the parent state of electron transfer and that nearly all electron transfer events lead to proton loss. Similar results regarding the parent state were obtained with a different electron acceptor, [Co(NH3)5Cl]2+; however, Stern-Volmer graphs revealed that [Co(NH3)5Cl]2+ was a more effective phosphorescence quencher (K SV = 230 000 M-1) compared to Cu(II)-azurin (K SV = 88 000 M-1). Competition experiments in the presence of both [Co(NH3)5Cl]2+ and Cu(II)-azurin suggested that [Co(NH3)5Cl]2+ is the preferred electron acceptor. Implications of these results in terms of quenching mechanisms are discussed.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources
