Multiple Facets of Modeling Electronic Absorption Spectra of Systems in Solution
- PMID: 36718266
- PMCID: PMC9881242
- DOI: 10.1021/acsphyschemau.2c00050
Multiple Facets of Modeling Electronic Absorption Spectra of Systems in Solution
Abstract
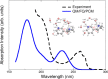
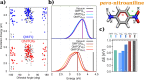
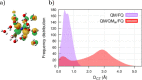
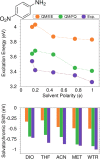
In this Perspective, we outline the essential physicochemical aspects that need to be considered when building a reliable approach to describe absorption properties of solvated systems. In particular, we focus on how to properly model the complexity of the solvation phenomenon, arising from dynamical aspects and specific, strong solute-solvent interactions. To this end, conformational and configurational sampling techniques, such as Molecular Dynamics, have to be coupled to accurate fully atomistic Quantum Mechanical/Molecular Mechanics (QM/MM) methodologies. By exploiting different illustrative applications, we show that an effective reproduction of experimental spectral signals can be achieved by delicately balancing exhaustive sampling, hydrogen bonding, mutual polarization, and nonelectrostatic effects.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
