The Use of Eculizumab for the Treatment of Atypical Hemolytic Uremic Syndrome in an Academic Hematology Center
- PMID: 36718583
- PMCID: PMC10266857
- DOI: 10.7812/TPP/22.073
The Use of Eculizumab for the Treatment of Atypical Hemolytic Uremic Syndrome in an Academic Hematology Center
Abstract
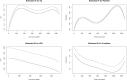
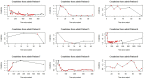
Introduction Eculizumab is a complement inhibitor used in treating atypical hemolytic uremic syndrome (aHUS). This study showcases patient demographics, clinical and laboratory results of these patients, and overall outcomes of patients with aHUS treated with eculizumab. Methods The authors conducted a retrospective case study including 9 patients who received at least 1 dose of eculizumab for treating aHUS. A linear mixed effects model was used with random effects for each patient and fixed effects for eculizumab and time since admission. A p value < 0.05 was significant. Results Nine patients were treated with eculizumab for aHUS. Most patients were Black (n = 5) with either Medicare or Medicaid (n = 5). Genetic mutations were tested for in 5 patients. There were significant decreases in lactate dehydrogenase (LD, p = 0.029) and creatinine (Cr, p = 0.012) when on treatment. No significance was found in hemoglobin (p = 0.258) or platelets (p = 0.569). Treatment was stopped in 7 patients, of which 3 had no evidence of disease relapse. The only adverse event was severe thrombocytopenia (n = 1). Discussion This multicase study is the first of its kind in which most patients are Black, showing that there is a lack of research of this kind, especially on genetic mutations. Most of our patients did not have private insurance or had Medicaid/Medicare. There was a 246.5-day median duration of treatment. There was low risk of adverse events. Conclusion This case series elucidates the effective use of eculizumab for atypical hemolytic uremic syndromein a diverse patient population and emphasizes the need for more research in this area.
Conflict of interest statement
Conflicts of InterestNone declared
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

