Disease progression modeling of the North Star Ambulatory Assessment for Duchenne Muscular Dystrophy
- PMID: 36718719
- PMCID: PMC10014057
- DOI: 10.1002/psp4.12921
Disease progression modeling of the North Star Ambulatory Assessment for Duchenne Muscular Dystrophy
Abstract
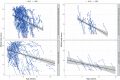
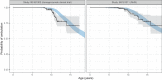
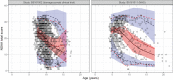
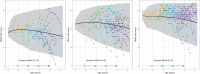
Duchenne muscular dystrophy (DMD) is a rare genetic disorder caused by decreased or absent dystrophin gene leading to progressive muscle degeneration and weakness in young boys. Disease progression models for the North Star Ambulatory Assessment (NSAA), a functional measurement widely used to assess outcomes in clinical trials, were developed using a longitudinal population modeling approach. The relationship between NSAA total score over time, loss of ambulation, and potential covariates that may influence disease progression were evaluated. Data included individual participant observations from an internal placebo-controlled phase II clinical trial and from the external natural history database for male patients with DMD obtained through the Cooperative International Neuromuscular Research Group (CINRG). A modified indirect response model for NSAA joined to a loss of ambulation (LOA) time-to-event model described the data well. Age was used as the independent variable because ambulatory function is known to vary with age. The NSAA and LOA models were linked using the dissipation rate constant parameter from the NSAA model by including the parameter as a covariate on the hazard equation for LOA. No covariates were identified. The model was then used as a simulation tool to explore various clinical trial design scenarios. This model contributes to the quantitative understanding of disease progression in DMD and may guide model-informed drug development decisions for ongoing and future DMD clinical trials.
© 2023 Pfizer Inc. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
J.E.H., P.J., and S.N. are employees of Pfizer Inc. and own stock or stock options in the company. L.O.H. is a former employee of Pfizer Inc. and owns stock in Pfizer Inc. As an Editor‐in‐Training for
Figures

References
-
- Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919‐928. - PubMed
-
- Daack‐Hirsch S, Holtzer C, Cunniff C. Parental perspectives on the diagnostic process for Duchenne and Becker muscular dystrophy. Am J Med Genet A. 2013;161A:687‐695. - PubMed
-
- Holtzer C, Meaney FJ, Andrews J, et al. Disparities in the diagnostic process of Duchenne and Becker muscular dystrophy. Genet Med. 2011;13:942‐947. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

