SPRTN-dependent DPC degradation precedes repair of damaged DNA: a proof of concept revealed by the STAR assay
- PMID: 36718861
- PMCID: PMC10085693
- DOI: 10.1093/nar/gkad022
SPRTN-dependent DPC degradation precedes repair of damaged DNA: a proof of concept revealed by the STAR assay
Abstract
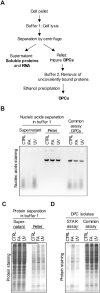
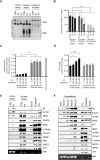
DNA-protein crosslinks (DPCs), formed by the covalent conjugation of proteins to DNA, are toxic lesions that interfere with DNA metabolic processing and transcription. The development of an accurate biochemical assay for DPC isolation is a priority for the mechanistic understanding of their repair. Here, we propose the STAR assay for the direct quantification of DPCs, sensitive to physiologically relevant treatment conditions. Implementing the STAR assay revealed the formation of small cross-linked peptides on DNA, created by the proteolytic degradation of DPCs by SPRTN. The initial proteolytic degradation of DPCs is required for the downstream activation of DNA repair, which is mediated through the phosphorylation of H2Ax. This leads to the accumulation of DNA repair factors on chromatin and the subsequent complete removal of the cross-linked peptides. These results confirmed that the repair of DPCs is a two-step process, starting with proteolytic resection by SPRTN, followed by the repair of the underlying damage to the DNA.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
-
- Smith K.C. Dose dependent decrease in extractability of DNA from bacteria following irradiation with ultraviolet light or with visible light plus dye. Biochem. Biophys. Res. Commun. 1962; 8:157–163. - PubMed
-
- Ide H., Nakano T., Shoulkamy M.I., Salem A.M.H.. Formation, repair, and biological effects of DNA–Protein cross-link damage. Advances in DNA Repair. InTech. 2015; 43–80.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

