Polarity Transduction Enables the Formal Electronically Mismatched Radical Addition to Alkenes
- PMID: 36718934
- PMCID: PMC9912259
- DOI: 10.1021/jacs.2c12699
Polarity Transduction Enables the Formal Electronically Mismatched Radical Addition to Alkenes
Abstract
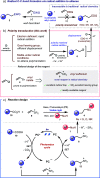
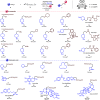
The formation of carbon-carbon bonds via the intermolecular addition of alkyl radicals to alkenes is a cornerstone of organic chemistry and plays a central role in synthesis. However, unless specific electrophilic radicals are involved, polarity matching requirements restrict the alkene component to be electron deficient. This limits the scope of a fundamentally important carbon-carbon bond forming process that could otherwise be more universally applied. Herein, we introduce a polarity transduction strategy that formally overcomes this electronic limitation. Vinyl sulfonium ions are demonstrated to react with carbon-centered radicals, giving adducts that undergo in situ or sequential nucleophilic displacement to provide products that would be inaccessible via traditional methods. The broad generality of this strategy is demonstrated through the derivatization of unmodified complex bioactive molecules.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
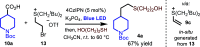
References
-
- Giese B. Formation of CC Bonds by Addition of Free Radicals to Alkenes. Angew. Chem., Int. Ed. Engl. 1983, 22, 753–764. 10.1002/anie.198307531. - DOI
-
- Encyclopedia of Radicals in Chemistry, Biology and Materials; Studer A., Chatgilialoglu C., Eds.; Wiley, 2012.
- Subramanian H.; Landais Y.; Sibi M. P.Radical Addition Reactions. In Comprehensive Organic Synthesis, 2nd ed.; Knochel P., Ed.; Elsevier, 2014; Vol. 4, pp 699–741.
- Srikanth G. S. C.; Castle S. L. Advances in Radical Conjugate Additions. Tetrahedron 2005, 61, 10377–10441. 10.1016/j.tet.2005.07.077. - DOI
- Streuff J.; Gansäuer A. Metal-Catalyzed β-Functionalization of Michael Acceptors through Reductive Radical Addition Reactions. Angew. Chem., Int. Ed. 2015, 54, 14232–14242. 10.1002/anie.201505231. - DOI - PubMed
-
- Curran D. P.; Porter N. A.; Giese B.. Stereochemistry of Radical Reactions; VCH: Weinheim, Germany, 1995.
- Sibi M. P.; Ji J. Practical and Efficient Enantioselective Conjugate Radical Additions. J. Org. Chem. 1997, 62, 3800–3801. 10.1021/jo970558y. - DOI
- Sibi M. P.; Porter N. A. Enantioselective Free Radical Reactions. Acc. Chem. Res. 1999, 32, 163–171. 10.1021/ar9600547. - DOI
- Sibi M. P.; Manyem S.; Zimmerman J. Enantioselective Radical Processes. Chem. Rev. 2003, 103, 3263–3296. 10.1021/cr020044l. - DOI - PubMed
- Bar G.; Parsons A. F. Stereoselective Radical Reactions. Chem. Soc. Rev. 2003, 32, 251–263. 10.1039/b111414j. - DOI - PubMed
-
- Jasperse C. P.; Curran D. P.; Fevig T. L. Radical Reactions in Natural Product Synthesis. Chem. Rev. 1991, 91, 1237–1286. 10.1021/cr00006a006. - DOI
- Pitre S. P.; Weires N. A.; Overman L. E. Forging C(Sp3)–C(Sp3) Bonds with Carbon-Centered Radicals in the Synthesis of Complex Molecules. J. Am. Chem. Soc. 2019, 141, 2800–2813. 10.1021/jacs.8b11790. - DOI - PMC - PubMed
- Nicholls T. P.; Leonori D.; Bissember A. C. Applications of Visible Light Photoredox Catalysis to the Synthesis of Natural Products and Related Compounds. Nat. Prod. Rep. 2016, 33, 1248–1254. 10.1039/C6NP00070C. - DOI - PubMed
-
- Nicewicz D. A.; MacMillan D. W. C. Merging Photoredox Catalysis with Organocatalysis: the Direct Asymmetric Alkylation of Aldehydes. Science 2008, 322, 77–80. 10.1126/science.1161976. - DOI - PMC - PubMed
- Ischay M. A.; Anzovino M. E.; Du J.; Yoon T. P. Efficient Visible Light Photocatalysis of [2 + 2] Enone Cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. 10.1021/ja805387f. - DOI - PubMed
- Narayanam J. M. R.; Tucker J. W.; Stephenson C. R. J. Electron-Transfer Photoredox Catalysis: Development of a Tin-Free Reductive Dehalogenation Reaction. J. Am. Chem. Soc. 2009, 131, 8756–8757. 10.1021/ja9033582. - DOI - PubMed
LinkOut - more resources
Full Text Sources

