Cigarette smoke depletes alveolar macrophages and delays clearance of Legionella pneumophila
- PMID: 36719079
- PMCID: PMC10026984
- DOI: 10.1152/ajplung.00268.2022
Cigarette smoke depletes alveolar macrophages and delays clearance of Legionella pneumophila
Abstract
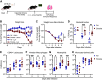
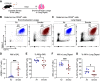
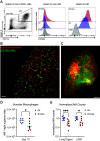
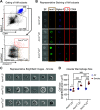
Legionella pneumophila is the main etiological agent of Legionnaires' disease, a severe bacterial pneumonia. L. pneumophila is initially engulfed by alveolar macrophages (AMs) and subvert normal cellular functions to establish a replicative vacuole. Cigarette smokers are particularly susceptible to developing Legionnaires' disease and other pulmonary infections; however, little is known about the cellular mechanisms underlying this susceptibility. To investigate this, we used a mouse model of acute cigarette smoke exposure to examine the immune response to cigarette smoke and subsequent L. pneumophila infection. Contrary to previous reports, we show that cigarette smoke exposure alone causes a significant depletion of AMs using enzymatic digestion to extract cells, or via imaging intact lung lobes by light-sheet microscopy. Furthermore, treatment of mice deficient in specific types of cell death with smoke suggests that NLRP3-driven pyroptosis is a contributor to smoke-induced death of AMs. After infection, smoke-exposed mice displayed increased pulmonary L. pneumophila loads and developed more severe disease compared with air-exposed controls. We tested if depletion of AMs was related to this phenotype by directly depleting them with clodronate liposomes and found that this also resulted in increased L. pneumophila loads. In summary, our results showed that cigarette smoke depleted AMs from the lung and that this likely contributed to more severe Legionnaires' disease. Furthermore, the role of AMs in L. pneumophila infection is more nuanced than simply providing a replicative niche, and our studies suggest they play a major role in bacterial clearance.
Keywords: Legionella pneumophila; alveolar macrophages; cigarette smoking; pyroptosis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

