Hypoxia-Inducible Factor 2α Attenuates Renal Ischemia-Reperfusion Injury by Suppressing CD36-Mediated Lipid Accumulation in Dendritic Cells in a Mouse Model
- PMID: 36719147
- PMCID: PMC10101615
- DOI: 10.1681/ASN.0000000000000027
Hypoxia-Inducible Factor 2α Attenuates Renal Ischemia-Reperfusion Injury by Suppressing CD36-Mediated Lipid Accumulation in Dendritic Cells in a Mouse Model
Abstract
Background: Hypoxia and hypoxia-inducible factors (HIFs) play essential and multiple roles in renal ischemia-reperfusion injury (IRI). Dendritic cells (DCs) comprise a major subpopulation of the immunocytes in the kidney and are key initiators and effectors of the innate immune responses after IRI. The role of HIF-2α in DCs remains unclear in the context of renal IRI.
Methods: To investigate the importance of HIF-2α in DCs upon renal IRI, we examined the effects of DC-specific HIF-2α ablation in a murine model. Bone marrow-derived DCs (BMDCs) from DC-specific HIF-2α-ablated mice and wild-type mice were used for functional studies and transcriptional profiling.
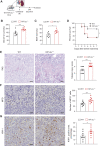
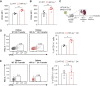
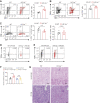
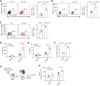
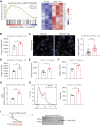
Results: DC-specific ablation of HIF-2α led to hyperactivation of natural killer T (NKT) cells, ultimately exacerbating murine renal IRI. HIF-2α deficiency in DCs triggered IFN-γ and IL-4 production in NKT cells, along with upregulation of type I IFN and chemokine responses that were critical for NKT cell activation. Mechanistically, loss of HIF-2α in DCs promoted their expression of CD36, a scavenger receptor for lipid uptake, increasing cellular lipid accumulation. Furthermore, HIF-2α bound directly to a reverse hypoxia-responsive element (rHRE) in the CD36 promoter. Importantly, CD36 blockade by sulfo-N-succinimidyl oleate (SSO) reduced NKT cell activation and abolished the exacerbation of renal IRI elicited by HIF-2α knockout.
Conclusions: Our study reveals a previously unrecognized role of the HIF-2α/CD36 regulatory axis in rewiring DC lipid metabolism under IRI-associated hypoxia. These findings suggest a potential therapeutic target to resolve long-standing obstacles in treatment of this severe complication.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
All authors have nothing to disclose.
Figures




References
-
- Smith SF, Hosgood SA, Nicholson ML: Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int 95: 50–56, 2019 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

