Roles for Microglia in Cryptococcal Brain Dissemination in the Zebrafish Larva
- PMID: 36719205
- PMCID: PMC10100726
- DOI: 10.1128/spectrum.04315-22
Roles for Microglia in Cryptococcal Brain Dissemination in the Zebrafish Larva
Abstract
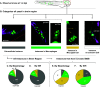
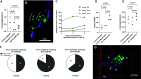
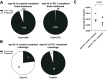
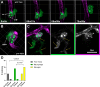
Cryptococcal infection begins in the lungs, but yeast cells subsequently access the bloodstream, from which they can reach the central nervous system (CNS). The resulting meningoencephalitis is the most common presentation and is very difficult to treat. How this fungus interacts with the blood-brain barrier (BBB) and establishes growth in the brain parenchyma remains a central question in fungal pathogenesis. We and others have developed the zebrafish larva as a model host for cryptococcosis and demonstrated that hematogenous CNS infection is replicated in this model. Here, we have used this model to examine the details of BBB crossing and the events immediately before and after. We have observed multiple mechanisms of BBB crossing and found that microglia, the resident phagocytes of the brain, likely have multiple roles. First, microglia either actively transfer yeast cells across the BBB or take up a significant proportion of them immediately after crossing. Second, microglia are capable of clearing individual cryptococcal cells at a developmental stage before adaptive immune cells have emerged. Third, microglia serve to maintain endothelial integrity, preventing other, phagocyte-independent forms of crossing. These proposed microglial functions during infection in the zebrafish larva generate new hypotheses concerning the establishment and control of cryptococcal meningoencephalitis. IMPORTANCE Cryptococcal meningitis is a fungal infection of the brain and a major cause of death in people with uncontrolled HIV. Infection begins in the lungs but can enter the bloodstream and disseminate to the brain. A structure called the blood-brain barrier must be crossed for the fungus to enter and cause meningitis. Learning how Cryptococcus crosses the blood-brain barrier will be crucial to understanding and possibly preventing brain infection. Using the zebrafish larva as a model host, we show that microglia, the resident phagocytes of the brain, potentially play multiple previously unappreciated roles in cryptococcal infection of the brain. These roles include reinforcing the integrity of the blood-brain barrier, clearing cryptococcal cells after they have crossed, and possibly participating directly in crossing via a previously unknown mechanism.
Keywords: Cryptococcus; blood-brain barrier; endothelial cells; host-pathogen interactions; macrophages; microglia; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kim JC, Crary B, Chang YC, Kwon-Chung KJ, Kim KJ. 2012. Cryptococcus neoformans activates RhoGTPase proteins followed by protein kinase C, focal adhesion kinase, and ezrin to promote traversal across the blood-brain barrier. J Biol Chem 287:36147–36157. doi: 10.1074/jbc.M112.389676. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

