Sirtuin-2, NAD-Dependent Deacetylase, Is a New Potential Therapeutic Target for HIV-1 Infection and HIV-Related Neurological Dysfunction
- PMID: 36719240
- PMCID: PMC9972991
- DOI: 10.1128/jvi.01655-22
Sirtuin-2, NAD-Dependent Deacetylase, Is a New Potential Therapeutic Target for HIV-1 Infection and HIV-Related Neurological Dysfunction
Abstract
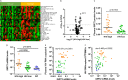
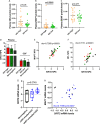
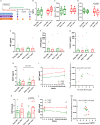
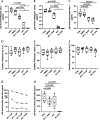
The implementation and access to combined antiretroviral treatment (cART) have dramatically improved the quality of life of people living with HIV (PLWH). However, some comorbidities, such as neurological disorders associated with HIV infection still represent a serious clinical challenge. Soluble factors in plasma that are associated with control of HIV replication and neurological dysfunction could serve as early biomarkers and as new therapeutic targets for this comorbidity. We used a customized antibody array for determination of blood plasma factors in 40 untreated PLWH with different levels of viremia and found sirtuin-2 (SIRT2), an NAD-dependent deacetylase, to be strongly associated with elevated viral loads and HIV provirus levels, as well as with markers of neurological damage (a-synuclein [SNCA], brain-derived neurotrophic factor [BDNF], microtubule-associated protein tau [MAPT], and neurofilament light protein [NFL]). Also, longitudinal analysis in HIV-infected individuals with immediate (n = 9) or delayed initiation (n = 10) of cART revealed that after 1 year on cART, SIRT2 plasma levels differed between both groups and correlated inversely with brain orbitofrontal cortex involution. Furthermore, targeting SIRT2 with specific small-molecule inhibitors in in vitro systems using J-LAT A2 and primary glial cells led to diminished HIV replication and virus reactivation from latency. Our data thus identify SIRT2 as a novel biomarker of uncontrolled HIV infection, with potential impact on neurological dysfunction and offers a new therapeutic target for HIV treatment and cure. IMPORTANCE Neurocognitive disorders are frequently reported in people living with HIV (PLWH) even with the introduction of combined antiretroviral treatment (cART). To identify biomarkers and potential therapeutic tools to target HIV infection in peripheral blood and in the central nervous system (CNS), plasma proteomics were applied in untreated chronic HIV-infected individuals with different levels of virus control. High plasma levels of sirtuin-2 (SIRT2), an NAD+ deacetylase, were detected in uncontrolled HIV infection and were strongly associated with plasma viral load and proviral levels. In parallel, SIRT2 levels in the peripheral blood and CNS were associated with markers of neurological damage and brain involution and were more pronounced in individuals who initiated cART later in infection. In vitro infection experiments using specific SIRT2 inhibitors suggest that specific targeting of SIRT2 could offer new therapeutic treatment options for HIV infections and their associated neurological dysfunction.
Keywords: HIV reservoir; HIV-1; HIV-associated neurocognitive disorders (HAND); neuroimaging; neurological disorder; plasma proteomics; virus infection control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Calcagno A, Atzori C, Romito A, Vai D, Audagnotto S, Stella ML, Montrucchio C, Imperiale D, Di Perri G, Bonora S. 2016. Blood brain barrier impairment is associated with cerebrospinal fluid markers of neuronal damage in HIV-positive patients. J Neurovirol 22:88–92. 10.1007/s13365-015-0371-x. - DOI - PubMed
-
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group . 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: charter study. Neurology 75:2087–2096. 10.1212/WNL.0b013e318200d727. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

