Next-generation antigen-presenting cell immune therapeutics for gliomas
- PMID: 36719372
- PMCID: PMC9888388
- DOI: 10.1172/JCI163449
Next-generation antigen-presenting cell immune therapeutics for gliomas
Abstract
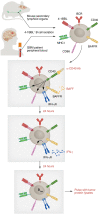
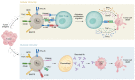
Antigen presentation machinery and professional antigen-presenting cells (APCs) are fundamental for an efficacious immune response against cancers, especially in the context of T cell-centric immunotherapy. Dendritic cells (DCs), the gold standard APCs, play a crucial role in initiating and maintaining a productive antigen-specific adaptive immunity. In recent decades, ex vivo-differentiated DCs from circulating CD14+ monocytes have become the reference for APC-based immunotherapy. DCs loaded with tumor-associated antigens, synthetic peptides, or RNA activate T cells with antitumor properties. This strategy has paved the way for the development of alternative antigen-presenting vaccination strategies, such as monocytes, B cells, and artificial APCs, that have shown effective therapeutic outcomes in preclinical cancer models. The search for alternative APC platforms was initiated by the overall limited clinical impact of DC vaccines, especially in indications such as gliomas, a primary brain tumor known for resistance to any immune intervention. In this Review, we navigate the APC immune therapeutics' past, present, and future in the context of primary brain tumors.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

