Neutrophil CEACAM1 determines susceptibility to NETosis by regulating the S1PR2/S1PR3 axis in liver transplantation
- PMID: 36719377
- PMCID: PMC9888387
- DOI: 10.1172/JCI162940
Neutrophil CEACAM1 determines susceptibility to NETosis by regulating the S1PR2/S1PR3 axis in liver transplantation
Abstract
Neutrophils, the largest innate immune cell population in humans, are the primary proinflammatory sentinel in the ischemia-reperfusion injury (IRI) mechanism in orthotopic liver transplantation (OLT). Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1, CC1, or CD66a) is essential in neutrophil activation and serves as a checkpoint regulator of innate immune-driven IRI cascade in OLT. Although CC1 alternative splicing generates two functionally distinct short and long cytoplasmic isoforms, their role in neutrophil activation remains unknown. Here, we undertook molecular and functional studies to interrogate the significance of neutrophil CC1 signaling in mouse and human OLT recipients. In the experimental arm, we employed a mouse OLT model to document that ablation of recipient-derived neutrophil CC1-long (CC1-L) isotype aggravated hepatic IRI by promoting neutrophil extracellular traps (NETs). Notably, by regulating the S1P-S1PR2/S1PR3 axis, neutrophil CC1-L determined susceptibility to NET formation via autophagy signaling. In the clinical arm, liver grafts from 55 transplant patients selectively enriched for neutrophil CC1-L showed relative resistance to ischemia-reperfusion (IR) stress/tissue damage, improved hepatocellular function, and clinical outcomes. In conclusion, despite neutrophils being considered a principal villain in peritransplant tissue injury, their CC1-L isoform may serve as a regulator of IR stress resistance/NETosis in human and mouse OLT recipients.
Keywords: Hypoxia; Neutrophils; Organ transplantation; Transplantation.
Figures
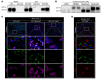


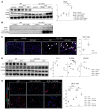
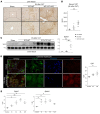
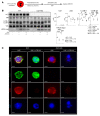
Comment in
- Avoid being trapped by your liver: Ischemia-reperfusion injury in liver transplant triggers SP1-mediated NETosis
Similar articles
-
Hepatic CEACAM1 expression indicates donor liver quality and prevents early transplantation injury.J Clin Invest. 2020 May 1;130(5):2689-2704. doi: 10.1172/JCI133142. J Clin Invest. 2020. PMID: 32027621 Free PMC article.
-
T Cell CEACAM1-TIM-3 Crosstalk Alleviates Liver Transplant Injury in Mice and Humans.Gastroenterology. 2023 Nov;165(5):1233-1248.e9. doi: 10.1053/j.gastro.2023.07.004. Epub 2023 Jul 19. Gastroenterology. 2023. PMID: 37479191 Free PMC article.
-
Avoid being trapped by your liver: ischemia-reperfusion injury in liver transplant triggers S1P-mediated NETosis.J Clin Invest. 2023 Feb 1;133(3):e167012. doi: 10.1172/JCI167012. J Clin Invest. 2023. PMID: 36719374 Free PMC article.
-
CD4+ T Cell NRF2 Signaling Improves Liver Transplantation Outcomes by Modulating T Cell Activation and Differentiation.Antioxid Redox Signal. 2023 Mar;38(7-9):670-683. doi: 10.1089/ars.2022.0094. Epub 2023 Mar 1. Antioxid Redox Signal. 2023. PMID: 36070449 Free PMC article. Review.
-
The Role of Neutrophils as a Driver in Hepatic Ischemia-Reperfusion Injury and Cancer Growth.Front Immunol. 2022 Jul 1;13:887565. doi: 10.3389/fimmu.2022.887565. eCollection 2022. Front Immunol. 2022. PMID: 35844608 Free PMC article. Review.
Cited by
-
Inhibition of CEACAM1 expression in cytokine-activated neutrophils using JAK inhibitors.BMC Immunol. 2024 Oct 1;25(1):63. doi: 10.1186/s12865-024-00656-6. BMC Immunol. 2024. PMID: 39354368 Free PMC article.
-
CEACAM1 increased the lymphangiogenesis through miR-423-5p and NF- kB in Non-Small Cell Lung Cancer.Biochem Biophys Rep. 2024 Sep 26;40:101833. doi: 10.1016/j.bbrep.2024.101833. eCollection 2024 Dec. Biochem Biophys Rep. 2024. PMID: 39398537 Free PMC article.
-
Alternative splicing of CEACAM1 by hypoxia-inducible factor-1α enhances tolerance to hepatic ischemia in mice and humans.Sci Transl Med. 2023 Aug 2;15(707):eadf2059. doi: 10.1126/scitranslmed.adf2059. Epub 2023 Aug 2. Sci Transl Med. 2023. PMID: 37531413 Free PMC article.
-
Lactate-Induced Mitochondrial Calcium Uptake 3 Aggravates Myocardial Ischemia-Reperfusion Injury by Promoting Neutrophil Extracellular Trap Formation.Research (Wash D C). 2025 May 30;8:0705. doi: 10.34133/research.0705. eCollection 2025. Research (Wash D C). 2025. PMID: 40452820 Free PMC article.
-
Mechanism and function of CEACAM1 splice isoforms.Eur J Clin Invest. 2024 Dec;54 Suppl 2(Suppl 2):e14350. doi: 10.1111/eci.14350. Eur J Clin Invest. 2024. PMID: 39674874 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

