PD-L1 translocation to the plasma membrane enables tumor immune evasion through MIB2 ubiquitination
- PMID: 36719382
- PMCID: PMC9888393
- DOI: 10.1172/JCI160456
PD-L1 translocation to the plasma membrane enables tumor immune evasion through MIB2 ubiquitination
Abstract
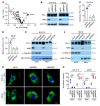
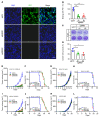
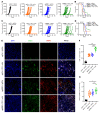
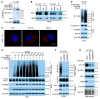
Programmed death-ligand 1 (PD-L1), a critical immune checkpoint ligand, is a transmembrane protein synthesized in the endoplasmic reticulum of tumor cells and transported to the plasma membrane to interact with programmed death 1 (PD-1) expressed on T cell surface. This interaction delivers coinhibitory signals to T cells, thereby suppressing their function and allowing evasion of antitumor immunity. Most companion or complementary diagnostic devices for assessing PD-L1 expression levels in tumor cells used in the clinic or in clinical trials require membranous staining. However, the mechanism driving PD-L1 translocation to the plasma membrane after de novo synthesis is poorly understood. Herein, we showed that mind bomb homolog 2 (MIB2) is required for PD-L1 transportation from the trans-Golgi network (TGN) to the plasma membrane of cancer cells. MIB2 deficiency led to fewer PD-L1 proteins on the tumor cell surface and promoted antitumor immunity in mice. Mechanistically, MIB2 catalyzed nonproteolytic K63-linked ubiquitination of PD-L1, facilitating PD-L1 trafficking through Ras-associated binding 8-mediated (RAB8-mediated) exocytosis from the TGN to the plasma membrane, where it bound PD-1 extrinsically to prevent tumor cell killing by T cells. Our findings demonstrate that nonproteolytic ubiquitination of PD-L1 by MIB2 is required for its transportation to the plasma membrane and tumor cell immune evasion.
Keywords: Cancer; Cancer immunotherapy; Cell Biology; Cellular immune response; Oncology.
Figures
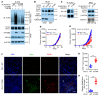
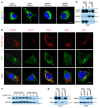
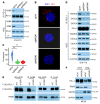
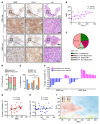
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

