Voice-Based Screening for SARS-CoV-2 Exposure in Cardiovascular Clinics (VOICE-COVID-19-II): Protocol for a Randomized Controlled Trial
- PMID: 36719720
- PMCID: PMC9891354
- DOI: 10.2196/41209
Voice-Based Screening for SARS-CoV-2 Exposure in Cardiovascular Clinics (VOICE-COVID-19-II): Protocol for a Randomized Controlled Trial
Erratum in
-
Authorship Correction: Voice-Based Screening for SARS-CoV-2 Exposure in Cardiovascular Clinics (VOICE-COVID-19-II): Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2023 Nov 23;12:e50893. doi: 10.2196/50893. JMIR Res Protoc. 2023. PMID: 37995337 Free PMC article.
Abstract
Background: The COVID-19 pandemic has disrupted the health care system, limiting health care resources such as the availability of health care professionals, patient monitoring, contact tracing, and continuous surveillance. As a result of this significant burden, digital tools have become an important asset in increasing the efficiency of patient care delivery. Digital tools can help support health care institutions by tracking transmission of the virus, aiding in the screening process, and providing telemedicine support. However, digital health tools face challenges associated with barriers to accessibility, efficiency, and privacy-related ethical issues.
Objective: This paper describes the study design of an open-label, noninterventional, crossover, randomized controlled trial aimed at assessing whether interactive voice response systems can screen for SARS-CoV-2 in patients as accurately as standard screening done by people. The study aims to assess the concordance and interrater reliability of symptom screening done by Amazon Alexa compared to manual screening done by research coordinators. The perceived level of comfort of patients when interacting with voice response systems and their personal experience will also be evaluated.
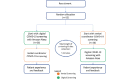
Methods: A total of 52 patients visiting the heart failure clinic at the Royal Victoria Hospital of the McGill University Health Center, in Montreal, Quebec, will be recruited. Patients will be randomly assigned to first be screened for symptoms of SARS-CoV-2 either digitally, by Amazon Alexa, or manually, by the research coordinator. Participants will subsequently be crossed over and screened either digitally or manually. The clinical setup includes an Amazon Echo Show, a tablet, and an uninterrupted power supply mounted on a mobile cart. The primary end point will be the interrater reliability on the accuracy of randomized screening data performed by Amazon Alexa versus research coordinators. The secondary end point will be the perceived level of comfort and app engagement of patients as assessed using 5-point Likert scales and binary mode responses.
Results: Data collection started in May 2021 and is expected to be completed in fall 2022. Data analysis is expected to be completed in early 2023.
Conclusions: The use of voice-based assistants could improve the provision of health services and reduce the burden on health care personnel. Demonstrating a high interrater reliability between Amazon Alexa and health care coordinators may serve future digital tools to streamline the screening and delivery of care in the context of other conditions and clinical settings. The COVID-19 pandemic occurs during the first digital era using digital tools such as Amazon Alexa for disease screening, and it represents an opportunity to implement such technology in health care institutions in the long term.
Trial registration: ClinicalTrials.gov NCT04508972; https://clinicaltrials.gov/ct2/show/NCT04508972.
International registered report identifier (irrid): DERR1-10.2196/41209.
Keywords: Alexa; Amazon; COVID-19; SARS-CoV-2; digital screening; voice-based technologies.
©Emily Oulousian, Seok Hoon Chung, Elie Ganni, Amir Razaghizad, Guang Zhang, Robert Avram, Abhinav Sharma. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 31.01.2023.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- WHO coronavirus (COVID-19) dashboard. World Health Organization. [2022-12-21]. https://covid19.who.int .
-
- Sadeghi S, Barzi A, Zarrin-Khameh N. Decision support system for medical triage. Stud Health Technol Inform. 2001;81:440–2. - PubMed
-
- Gunasekeran DV, Tseng RMWW, Tham Y, Wong TY. Applications of digital health for public health responses to COVID-19: a systematic scoping review of artificial intelligence, telehealth and related technologies. NPJ Digit Med. 2021 Feb 26;4(1):40. doi: 10.1038/s41746-021-00412-9. doi: 10.1038/s41746-021-00412-9.10.1038/s41746-021-00412-9 - DOI - PMC - PubMed
-
- Luk TT, Lui JHT, Wang MP. Efficacy, usability, and acceptability of a chatbot for promoting COVID-19 vaccination in unvaccinated or booster-hesitant young adults: pre-post pilot study. J Med Internet Res. 2022 Oct 04;24(10):e39063. doi: 10.2196/39063. https://www.jmir.org/2022/10/e39063/ v24i10e39063 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous