Cellular Uptake of Bevacizumab in Cervical and Breast Cancer Cells Revealed by Single-Molecule Spectroscopy
- PMID: 36719904
- PMCID: PMC9923738
- DOI: 10.1021/acs.jpclett.2c03590
Cellular Uptake of Bevacizumab in Cervical and Breast Cancer Cells Revealed by Single-Molecule Spectroscopy
Abstract
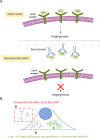
Bevacizumab is a biological drug that is now extensively studied in clinics against various types of cancer. Although bevacizumab's action is preferably extracellular, there are reports suggesting its internalization into cancer cells, consequently decreasing its therapeutic potential. Here we are solving this issue by applying fluorescence correlation spectroscopy in living cells. We tracked single molecules of fluorescent bevacizumab in MDA-MB-231 and HeLa cells and proved that mobility measurements bring significant added value to standard imaging techniques. We confirmed the presence of the drug in intracellular vesicles. Additionally, we explicitly excluded the presence of a free cytosolic fraction of bevacizumab in both studied cell types. Extracellular and intracellular concentrations of the drug were measured, giving a partition coefficient on the order of 10-5, comparable with the spontaneous uptake of biologically inert nanoparticles. Our work presents how techniques and models developed for physics can answer biological questions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- U.S. National Library of Medicine (accessed May 2022).
-
- Müller-Greven G.; Carlin C. R.; Burgett M. E.; Ahluwalia M. S.; Lauko A.; Nowacki A. S.; Herting C. J.; Qadan M. A.; Bredel M.; Toms S. A.; Lathia J. D.; Hambardzumyan D.; Sarkaria J. N.; Hamerlik P.; Gladson C. L. Macropinocytosis of Bevacizumab by Glioblastoma Cells in the Perivascular Niche Affects Their Survival. Clin. Cancer Res. 2017, 23 (22), 7059–7071. 10.1158/1078-0432.CCR-17-0249. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

