The dual-function chemokine receptor CCR2 drives migration and chemokine scavenging through distinct mechanisms
- PMID: 36719944
- PMCID: PMC10091583
- DOI: 10.1126/scisignal.abo4314
The dual-function chemokine receptor CCR2 drives migration and chemokine scavenging through distinct mechanisms
Abstract
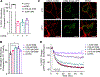
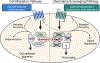
C-C chemokine receptor 2 (CCR2) is a dual-function receptor. Similar to other G protein-coupled chemokine receptors, it promotes monocyte infiltration into tissues in response to the chemokine CCL2, and, like atypical chemokine receptors (ACKRs), it scavenges chemokine from the extracellular environment. CCR2 therefore mediates CCL2-dependent signaling as a G protein-coupled receptor (GPCR) and also limits CCL2 signaling as a scavenger receptor. We investigated the mechanisms underlying CCR2 scavenging, including the involvement of intracellular proteins typically associated with GPCR signaling and internalization. Using CRISPR knockout cell lines, we showed that CCR2 scavenged by constitutively internalizing to remove CCL2 from the extracellular space and recycling back to the cell surface for further rounds of ligand sequestration. This process occurred independently of G proteins, GPCR kinases (GRKs), β-arrestins, and clathrin, which is distinct from other "professional" chemokine scavenger receptors that couple to GRKs, β-arrestins, or both. These findings set the stage for understanding the molecular regulators that determine CCR2 scavenging and may have implications for drug development targeting this therapeutically important receptor.
Conflict of interest statement
Figures



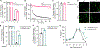


References
-
- Nibbs RJB, Graham GJ, Immune regulation by atypical chemokine receptors. Nat Rev Immunol 13, 815–829 (2013). - PubMed
-
- Abe P, Mueller W, Schütz D, MacKay F, Thelen M, Zhang P, Stumm R, CXCR7 prevents excessive CXCL12-mediated downregulation of CXCR4 in migrating cortical interneurons. Development 141, 1857–1863 (2014). - PubMed

