CD73-Dependent Adenosine Signaling through Adora2b Drives Immunosuppression in Ductal Pancreatic Cancer
- PMID: 36720042
- PMCID: PMC10071819
- DOI: 10.1158/0008-5472.CAN-22-2553
CD73-Dependent Adenosine Signaling through Adora2b Drives Immunosuppression in Ductal Pancreatic Cancer
Abstract
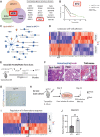
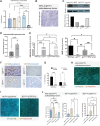
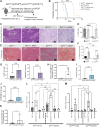
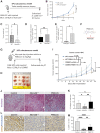
The microenvironment that surrounds pancreatic ductal adenocarcinoma (PDAC) is profoundly desmoplastic and immunosuppressive. Understanding triggers of immunosuppression during the process of pancreatic tumorigenesis would aid in establishing targets for effective prevention and therapy. Here, we interrogated differential molecular mechanisms dependent on cell of origin and subtype that promote immunosuppression during PDAC initiation and in established tumors. Transcriptomic analysis of cell-of-origin-dependent epithelial gene signatures revealed that Nt5e/CD73, a cell-surface enzyme required for extracellular adenosine generation, is one of the top 10% of genes overexpressed in murine tumors arising from the ductal pancreatic epithelium as opposed to those rising from acinar cells. These findings were confirmed by IHC and high-performance liquid chromatography. Analysis in human PDAC subtypes indicated that high Nt5e in murine ductal PDAC models overlaps with high NT5E in human PDAC squamous and basal subtypes, considered to have the highest immunosuppression and worst prognosis. Multiplex immunofluorescent analysis showed that activated CD8+ T cells in the PDAC tumor microenvironment express high levels of CD73, indicating an opportunity for immunotherapeutic targeting. Delivery of CD73 small-molecule inhibitors through various delivery routes reduced tumor development and growth in genetically engineered and syngeneic mouse models. In addition, the adenosine receptor Adora2b was a determinant of adenosine-mediated immunosuppression in PDAC. These findings highlight a molecular trigger of the immunosuppressive PDAC microenvironment elevated in the ductal cell of origin, linking biology with subtype classification, critical components for PDAC immunoprevention and personalized approaches for immunotherapeutic intervention.
Significance: Ductal-derived pancreatic tumors have elevated epithelial and CD8+GZM+ T-cell CD73 expression that confers sensitivity to small-molecule inhibition of CD73 or Adora2b to promote CD8+ T-cell-mediated tumor regression. See related commentary by DelGiorno, p. 977.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
-
CD73 Is a Critical Immune Checkpoint in a Molecular Subtype of Pancreatic Cancer.Cancer Res. 2023 Apr 4;83(7):977-978. doi: 10.1158/0008-5472.CAN-23-0268. Cancer Res. 2023. PMID: 37014046
References
-
- Katz MHG, Shi Q, Meyers J, Herman JM, Chuong M, Wolpin BM, et al. . Efficacy of preoperative mFOLFIRINOX vs mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: the A021501 phase 2 randomized clinical trial. JAMA Oncol 2022;8:1263–70. - PMC - PubMed
-
- Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Köhle C, et al. . Protective role of ecto-5'-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 2007;18:833–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

