Efficacy and safety of elinzanetant, a selective neurokinin-1,3 receptor antagonist for vasomotor symptoms: a dose-finding clinical trial (SWITCH-1)
- PMID: 36720081
- PMCID: PMC9970022
- DOI: 10.1097/GME.0000000000002138
Efficacy and safety of elinzanetant, a selective neurokinin-1,3 receptor antagonist for vasomotor symptoms: a dose-finding clinical trial (SWITCH-1)
Abstract
Objective: Neurokinin (NK)-3 and NK-1 receptors have been implicated in the etiology of vasomotor symptoms (VMS) and sleep disturbances associated with menopause. This phase 2b, adaptive, dose-range finding study aimed to assess the efficacy and safety of multiple doses of elinzanetant (NT-814), a selective NK-1,3 receptor antagonist, in women experiencing VMS associated with menopause, and investigate the impact of elinzanetant on sleep and quality of life.
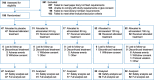
Methods: Postmenopausal women aged 40 to 65 years who experienced seven or more moderate-to-severe VMS per day were randomized to receive elinzanetant 40, 80, 120, or 160 mg or placebo once daily using an adaptive design algorithm. Coprimary endpoints were reduction in mean frequency and severity of moderate-to-severe VMS at weeks 4 and 12. Secondary endpoints included patient-reported assessments of sleep and quality of life.
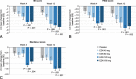
Results: Elinzanetant 120 mg and 160 mg achieved reductions in VMS frequency versus placebo from week 1 throughout 12 weeks of treatment. Least square mean reductions were statistically significant versus placebo at both primary endpoint time points for elinzanetant 120 mg (week 4: -3.93 [SE, 1.02], P < 0.001; week 12: -2.95 [1.15], P = 0.01) and at week 4 for elinzanetant 160 mg (-2.63 [1.03]; P = 0.01). Both doses also led to clinically meaningful improvements in measures of sleep and quality of life. All doses of elinzanetant were well tolerated.
Conclusions: Elinzanetant is an effective and well-tolerated nonhormone treatment option for postmenopausal women with VMS and associated sleep disturbance. Elinzanetant also improves quality of life in women with VMS.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The North American Menopause Society.
Conflict of interest statement
Financial disclosure/conflicts of interest: C.C. and L.Z. are employees of Bayer CC AG. C.S. is an employee of Bayer AG. M.K., S.P., E.B., S.S., and M.T. are employees of NeRRe Therapeutics. J.B., as an employee of Cytel Inc, was a paid statistical consultant on this trial. R.A.A. has undertaken consultancy work for NeRRe Therapeutics and Sojournix Inc. J.A.S. has grant/research support from AbbVie, Inc, Bayer Healthcare LLC, Daré Bioscience, Ipsen, Mylan/Viatris Inc, Myovant Sciences, ObsEva SA, Sebela Pharmaceuticals Inc, and Viveve Medical; has been a consultant/advisory boards of Bayer HealthCare Pharmaceuticals Inc, Besins Healthcare, California Institute of Integral Studies, Camargo Pharmaceutical Services, LLC, Covance Inc, Daré Bioscience, DEKA M.E.L.A. S.r.l., Femasys Inc, KaNDy/NeRRe Therapeutics Ltd, Khyria, Madorra Pty Ltd, Mitsubishi Tanabe Pharma Development America, Inc, QUE Oncology Pty, Limited, Scynexis Inc, Sebela Pharmaceuticals, Inc, Sprout Pharmaceuticals, Inc, and Vella Bioscience Inc; has served on the Speaker's bureaus of Mayne Pharma, Inc, Myovant Sciences, Inc, Pfizer Inc, Pharmavite LLC, Scynexis Inc, and TherapeuticsMD; and is a stockholder (direct purchase) in Sermonix Pharmaceuticals. N.P. has undertaken consultancy and speaker's bureau work for a number of pharmaceutical companies including Bayer AG. H.J. has received grant funding from National Institutes of Health, Merck, Pfizer, and Hello Therapeutics; has undertaken consultancy work for Bayer, Jazz, and Eisai; and her spouse is an employee at Arsenal Biosciences and has equity at Merck.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

