Lung-resident CD69+ST2+ TH2 cells mediate long-term type 2 memory to inhaled antigen in mice
- PMID: 36720287
- PMCID: PMC10330297
- DOI: 10.1016/j.jaci.2023.01.016
Lung-resident CD69+ST2+ TH2 cells mediate long-term type 2 memory to inhaled antigen in mice
Abstract
Background: Chronic airway diseases such as asthma are characterized by persistent type 2 immunity in the airways. We know little about the mechanisms that explain why type 2 inflammation continues in these diseases.
Objective: We used mouse models to investigate the mechanisms involved in long-lasting immune memory.
Methods: Naive mice were exposed intranasally to ovalbumin (OVA) antigen with Alternaria extract as an adjuvant. Type 2 memory was analyzed by parabiosis model, flow cytometry with in vivo antibody labeling, and intranasal OVA recall challenge. Gene-deficient mice were used to analyze the mechanisms.
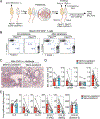
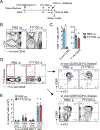
Results: In the parabiosis model, mice previously exposed intranasally to OVA with Alternaria showed more robust antigen-specific immune responses and airway inflammation than mice with circulating OVA-specific T cells. After a single airway exposure to OVA with Alternaria, CD69+ST2+ TH2-type T cells, which highly express type 2 cytokine messenger RNA and lack CD62L expression, appeared in lung tissue within 5 days and persisted for at least 84 days. When exposed again to OVA in vivo, these cells produced type 2 cytokines quickly without involving circulating T cells. Development of tissue-resident CD69+ST2+ TH2 cells and long-term memory to an inhaled antigen were abrogated in mice deficient in ST2 or IL-33, but not TSLP receptor.
Conclusion: CD69+ST2+ TH2 memory cells develop quickly in lung tissue after initial allergen exposure and persist for a prolonged period. The ST2/IL-33 pathway may play a role in the development of immune memory in lung to certain allergens.
Keywords: IL-33; IL-5; T(H)2 cells; allergens; allergy; tissue-resident memory T cells.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors acknowledge no conflict of interest related to this manuscript.
Figures





Similar articles
-
Respiratory syncytial virus prevents the subsequent development of ovalbumin-induced allergic responses by inhibiting ILC2 via the IL-33/ST2 pathway.Immunotherapy. 2018 Sep;10(12):1065-1076. doi: 10.2217/imt-2018-0059. Epub 2018 Jul 20. Immunotherapy. 2018. PMID: 30027786
-
ST2 requires Th2-, but not Th17-, type airway inflammation in epicutaneously antigen- sensitized mice.Allergol Int. 2012 Jun;61(2):265-73. doi: 10.2332/allergolint.11-OA-0379. Epub 2012 Feb 25. Allergol Int. 2012. PMID: 22361513
-
The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma.Clin Exp Allergy. 2016 Mar;46(3):479-90. doi: 10.1111/cea.12683. Clin Exp Allergy. 2016. PMID: 26609909
-
Louki Zupa decoction attenuates the airway inflammation in acute asthma mice induced by ovalbumin through IL-33/ST2-NF-κB/GSK3β/mTOR signalling pathway.Pharm Biol. 2022 Dec;60(1):1520-1532. doi: 10.1080/13880209.2022.2104327. Pharm Biol. 2022. PMID: 35952388 Free PMC article.
-
Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine.Cytokine. 2022 Aug;156:155891. doi: 10.1016/j.cyto.2022.155891. Epub 2022 May 25. Cytokine. 2022. PMID: 35640416 Review.
Cited by
-
TLR4+group 2 innate lymphoid cells contribute to persistent type 2 immunity in airway diseases.Nat Commun. 2025 Aug 2;16(1):7108. doi: 10.1038/s41467-025-62532-0. Nat Commun. 2025. PMID: 40753172 Free PMC article.
-
Tissue-Resident Memory T Cells in Allergy.Clin Rev Allergy Immunol. 2024 Feb;66(1):64-75. doi: 10.1007/s12016-024-08982-8. Epub 2024 Feb 21. Clin Rev Allergy Immunol. 2024. PMID: 38381299 Review.
-
Antiviral roles of eosinophils in asthma and respiratory viral infection.Front Allergy. 2025 Feb 28;6:1548338. doi: 10.3389/falgy.2025.1548338. eCollection 2025. Front Allergy. 2025. PMID: 40083723 Free PMC article. Review.
-
Update on type 2 immunity.J Allergy Clin Immunol. 2025 Feb;155(2):327-335. doi: 10.1016/j.jaci.2024.11.003. Epub 2024 Nov 9. J Allergy Clin Immunol. 2025. PMID: 39528097 Review.
-
Crystalline silica-induced recruitment and immuno-imbalance of CD4+ tissue resident memory T cells promote silicosis progression.Commun Biol. 2024 Aug 9;7(1):971. doi: 10.1038/s42003-024-06662-z. Commun Biol. 2024. PMID: 39122899 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

