Impairment of adrenergically-regulated thermogenesis in brown fat of obesity-resistant mice is compensated by non-shivering thermogenesis in skeletal muscle
- PMID: 36720306
- PMCID: PMC9922683
- DOI: 10.1016/j.molmet.2023.101683
Impairment of adrenergically-regulated thermogenesis in brown fat of obesity-resistant mice is compensated by non-shivering thermogenesis in skeletal muscle
Abstract
Objective: Non-shivering thermogenesis (NST) mediated by uncoupling protein 1 (UCP1) in brown adipose tissue (BAT) can be activated via the adrenergic system in response to cold or diet, contributing to both thermal and energy homeostasis. Other mechanisms, including metabolism of skeletal muscle, may also be involved in NST. However, relative contribution of these energy dissipating pathways and their adaptability remain a matter of long-standing controversy.
Methods: We used warm-acclimated (30 °C) mice to characterize the effect of an up to 7-day cold acclimation (6 °C; CA) on thermoregulatory thermogenesis, comparing inbred mice with a genetic background conferring resistance (A/J) or susceptibility (C57BL/6 J) to obesity.
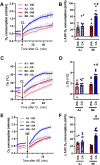
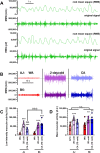
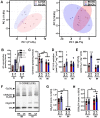
Results: Both warm-acclimated C57BL/6 J and A/J mice exhibited similar cold endurance, assessed as a capability to maintain core body temperature during acute exposure to cold, which improved in response to CA, resulting in comparable cold endurance and similar induction of UCP1 protein in BAT of mice of both genotypes. Despite this, adrenergic NST in BAT was induced only in C57BL/6 J, not in A/J mice subjected to CA. Cold tolerance phenotype of A/J mice subjected to CA was not based on increased shivering, improved insulation, or changes in physical activity. On the contrary, lipidomic, proteomic and gene expression analyses along with palmitoyl carnitine oxidation and cytochrome c oxidase activity revealed induction of lipid oxidation exclusively in skeletal muscle of A/J mice subjected to CA. These changes appear to be related to skeletal muscle NST, mediated by sarcolipin-induced uncoupling of sarco(endo)plasmic reticulum calcium ATPase pump activity and accentuated by changes in mitochondrial respiratory chain supercomplexes assembly.
Conclusions: Our results suggest that NST in skeletal muscle could be adaptively augmented in the face of insufficient adrenergic NST in BAT, depending on the genetic background of the mice. It may provide both protection from cold and resistance to obesity, more effectively than BAT.
Keywords: Brown adipose tissue; Mitochondrial supercomplex; Non-shivering thermogenesis; Obesity; Sarcolipin; Skeletal muscle.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures






References
-
- Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. - PubMed
-
- Jansky I. Humoral thermogenesis and its role in maintaining energy balance. Physiol Rev. 1995;75(2):237–259. - PubMed
-
- Silva J.E. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86(2):435–464. - PubMed
-
- Flachs P., Rossmeisl M., Kuda O., Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: a key to lean phenotype. Biochim Biophys Acta. 2013;1831(5):986–1003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

