Induction and subsequent decline of S1-specific T cell reactivity after COVID-19 vaccination
- PMID: 36720440
- PMCID: PMC9884141
- DOI: 10.1016/j.clim.2023.109248
Induction and subsequent decline of S1-specific T cell reactivity after COVID-19 vaccination
Abstract
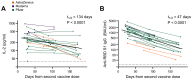
We analyzed magnitude and duration of SARS-CoV-2-specific T cell responses in healthy, infection-naïve subjects receiving COVID-19 vaccines. Overlapping peptides spanning the N-terminal spike 1 (S1) domain of the spike protein triggered secretion of the T cell-derived cytokine interleukin-2 ex vivo in 94/94 whole blood samples from vaccinated subjects at levels exceeding those recorded in all 45 pre-vaccination samples. S1-specific T cell reactivity was stronger in vaccinated subjects compared with subjects recovering from natural COVID-19 and decayed with an estimated half-life of 134 days in the first six months after the 2nd vaccination. We conclude that COVID-19 vaccination induces robust T cell immunity that subsequently declines. EudraCT 2021-000349-42. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2021-000349-42.
Keywords: COVID-19; Longevity; SARS-CoV-2; T cells; Vaccine.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures

References
-
- Guerrera G., Picozza M., D’Orso S., et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci Immunol. 2021;6:eabl5344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

