High-Performance Implantable Sensors based on Anisotropic Magnetoresistive La0.67Sr0.33MnO3 for Biomedical Applications
- PMID: 36720461
- PMCID: PMC9930082
- DOI: 10.1021/acsbiomaterials.2c01147
High-Performance Implantable Sensors based on Anisotropic Magnetoresistive La0.67Sr0.33MnO3 for Biomedical Applications
Abstract
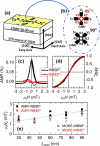
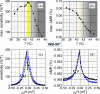
We present the design, fabrication, and characterization of an implantable neural interface based on anisotropic magnetoresistive (AMR) magnetic-field sensors that combine reduced size and high performance at body temperature. The sensors are based on La0.67Sr0.33MnO3 (LSMO) as a ferromagnetic material, whose epitaxial growth has been suitably engineered to get uniaxial anisotropy and large AMR output together with low noise even at low frequencies. The performance of LSMO sensors of different film thickness and at different temperatures close to 37 °C has to be explored to find an optimum sensitivity of ∼400%/T (with typical detectivity values of 2 nT·Hz-1/2 at a frequency of 1 Hz and 0.3 nT·Hz-1/2 at 1 kHz), fitted for the detection of low magnetic signals coming from neural activity. Biocompatibility tests of devices consisting of submillimeter-size LSMO sensors coated by a thin poly(dimethyl siloxane) polymeric layer, both in vitro and in vivo, support their high suitability as implantable detectors of low-frequency biological magnetic signals emerging from heterogeneous electrically active tissues.
Keywords: anisotropic magnetoresistance; detectivity measurements; in vitro and in vivo biocompatibility; magnetic sensors; neural interfaces; thin film oxide.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Dagotto E. Open questions in CMR manganites, relevance of clustered states and analogies with other compounds including the cuprates. New J. Phys. 2005, 7, 67. 10.1088/1367-2630/7/1/067. - DOI
-
- Han S. J.; Xu L.; Yu H.; Wilson R. J.; White R. L.; Pourmand N.; Wang S. X.. CMOS Integrated DNA Microarray Based on GMR Sensors International Electron Device Meeting San Francisco, CA, USA, 2006, pp. 1-4, 10.1109/IEDM.2006.346887. - DOI
-
- Mao S.; ChenLiu Y. F.; Chen X.; Xu B.; Lu P.; Patwari M.; Xi H.; Chang C.; Miller B.; Menard D.; Pant B.; Loven J.; Duxstad K.; Li S.; Zhang Z.; Johnston A.; Lamberton R.; Gubbins M.; McLauguin T.; Gadbois J.; Ding J.; Cross B.; Xue S.; Ryan P. IEEE Trans. Magn. 2006, 42, 97–102. 10.1109/tmag.2005.863772. - DOI
-
- Stutzke N. A.; Russek S. E.; Pappas D. P.; Tondra M. Low-frequency noise measurements on commercial magnetoresistive magnetic field sensors. J. Appl. Phys. 2005, 97, 10Q107. 10.1063/1.1861375. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

