Efficacy and safety of tofacitinib in Chinese patients with active psoriatic arthritis: a phase 3, randomised, double-blind, placebo-controlled study
- PMID: 36720560
- PMCID: PMC9890804
- DOI: 10.1136/rmdopen-2022-002559
Efficacy and safety of tofacitinib in Chinese patients with active psoriatic arthritis: a phase 3, randomised, double-blind, placebo-controlled study
Abstract
Objectives: Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, were evaluated in a 6-month, double-blind, phase 3 study in Chinese patients with active (polyarthritic) psoriatic arthritis (PsA) and inadequate response to ≥1 conventional synthetic disease-modifying antirheumatic drug.
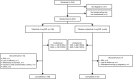
Methods: Patients were randomised (2:1) to tofacitinib 5 mg twice daily (N=136) or placebo (N=68); switched to tofacitinib 5 mg twice daily after month (M)3 (blinded).
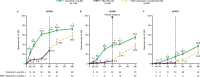
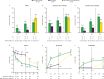
Primary endpoint: American College of Rheumatology (ACR50) response at M3. Secondary endpoints (through M6) included: ACR20/50/70 response; change from baseline in Health Assessment Questionnaire-Disability Index (HAQ-DI); ≥75% improvement in Psoriasis Area and Severity Index (PASI75) response, and enthesitis and dactylitis resolution. Safety was assessed throughout.
Results: The primary endpoint was met (tofacitinib 5 mg twice daily, 38.2%; placebo, 5.9%; p<0.0001). M3 ACR20/ACR70/PASI75 responses, and enthesitis and dactylitis resolution rates, were higher and HAQ-DI reduction was greater for tofacitinib 5 mg twice daily versus placebo. Incidence of adverse events (AEs)/serious AEs (M0-3): 68.4%/0%, tofacitinib 5 mg twice daily; 75.0%/4.4%, placebo. One death was reported with placebo→tofacitinib 5 mg twice daily (due to accident). One serious infection, non-serious herpes zoster, and lung cancer case each were reported with tofacitinib 5 mg twice daily; four serious infections and one non-serious herpes zoster case were reported with placebo→tofacitinib 5 mg twice daily (M0-6). No non-melanoma skin cancer, major adverse cardiovascular or thromboembolism events were reported.
Conclusion: In Chinese patients with PsA, tofacitinib efficacy was greater than placebo (primary and secondary endpoints). Tofacitinib was well tolerated; safety outcomes were consistent with the established safety profile in PsA and other indications.
Trial registration number: NCT03486457.
Keywords: Arthritis; Arthritis, Psoriatic; Inflammation.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: XZ, XL, WW, ZJ, YL, SL, ZhuZ, ZhiZ, JX, WT, JH, JiL and JuL declare no conflicts of interests. WL is a former employee of Pfizer Inc. SL, KK, CW, LMG, CK and OD are current employees and shareholders of Pfizer Inc.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
