Novel Genetic Variants Associated with Chronic Kidney Disease Progression
- PMID: 36720675
- PMCID: PMC10125649
- DOI: 10.1681/ASN.0000000000000066
Novel Genetic Variants Associated with Chronic Kidney Disease Progression
Erratum in
-
Correction: Incorrect Prepublished Article DOIs.J Am Soc Nephrol. 2023 Apr 1;34(4):723. doi: 10.1681/ASN.0000000000000080. Epub 2023 Feb 1. J Am Soc Nephrol. 2023. PMID: 36735808 Free PMC article. No abstract available.
Abstract
Significance statement: eGFR slope has been used as a surrogate outcome for progression of CKD. However, genetic markers associated with eGFR slope among patients with CKD were unknown. We aimed to identify genetic susceptibility loci associated with eGFR slope. A two-phase genome-wide association study identified single nucleotide polymorphisms (SNPs) in TPPP and FAT1-LINC02374 , and 22 of them were used to derive polygenic risk scores that mark the decline of eGFR by disrupting binding of nearby transcription factors. This work is the first to identify the impact of TPPP and FAT1-LINC02374 on CKD progression, providing predictive markers for the decline of eGFR in patients with CKD.
Background: The incidence of CKD is associated with genetic factors. However, genetic markers associated with the progression of CKD have not been fully elucidated.
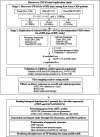
Methods: We conducted a genome-wide association study among 1738 patients with CKD, mainly from the KoreaN cohort study for Outcomes in patients With CKD. The outcome was eGFR slope. We performed a replication study for discovered single nucleotide polymorphisms (SNPs) with P <10 -6 in 2498 patients with CKD from the Chronic Renal Insufficiency Cohort study. Several expression quantitative trait loci (eQTL) studies, pathway enrichment analyses, exploration of epigenetic architecture, and predicting disruption of transcription factor (TF) binding sites explored potential biological implications of the loci. We developed and evaluated the effect of polygenic risk scores (PRS) on incident CKD outcomes.
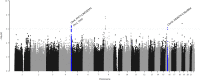
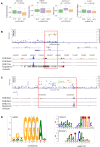
Results: SNPs in two novel loci, TPPP and FAT1-LINC02374 , were replicated (rs59402340 in TPPP , Pdiscovery =7.11×10 -7 , PCRIC =8.13×10 -4 , Pmeta =7.23×10 -8 ; rs28629773 in FAT1-LINC02374 , Pdiscovery =6.08×10 -7 , PCRIC =4.33×10 -2 , Pmeta =1.87×10 -7 ). The eQTL studies revealed that the replicated SNPs regulated the expression level of nearby genes associated with kidney function. Furthermore, these SNPs were near gene enhancer regions and predicted to disrupt the binding of TFs. PRS based on the independently significant top 22 SNPs were significantly associated with CKD outcomes.
Conclusions: This study demonstrates that SNP markers in the TPPP and FAT1-LINC02374 loci could be predictive markers for the decline of eGFR in patients with CKD.
Copyright © 2023 by the American Society of Nephrology.
Figures



Comment in
-
Genetic Association Analysis of Chronic Kidney Disease Progression in a Small Korean Cohort Study.J Am Soc Nephrol. 2023 May 1;34(5):729-731. doi: 10.1681/ASN.0000000000000110. J Am Soc Nephrol. 2023. PMID: 37126668 Free PMC article. No abstract available.
References
-
- Oshima M, Jun M, Ohkuma T, et al. ; on behalf of the ADVANCE Collaborative Group. The relationship between eGFR slope and subsequent risk of vascular outcomes and all-cause mortality in type 2 diabetes: the ADVANCE-ON study. Diabetologia. 2019;62(11):1988–1997. doi: 10.1007/s00125-019-4948-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

