A Bidentate Ligand Featuring Ditopic Lewis Acids in the Second Sphere for Selective Substrate Capture and Activation
- PMID: 36720708
- PMCID: PMC10023486
- DOI: 10.1002/anie.202218907
A Bidentate Ligand Featuring Ditopic Lewis Acids in the Second Sphere for Selective Substrate Capture and Activation
Abstract
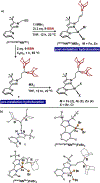
We present a ligand platform featuring appended ditopic Lewis acids to facilitate capture/activation of diatomic substrates. We show that incorporation of two 9-borabicyclo[3.3.1]nonane (9-BBN) units on a single carbon tethered to a pyridine pyrazole scaffold maintains a set of unquenched nitrogen donors available to coordinate FeII , ZnII , and NiII . Using hydride ion affinity and competition experiments, we establish an additive effect for ditopic secondary sphere boranes, compared to the monotopic analogue. These effects are exploited to achieve high selectivity for binding NO2 - in the presence of competitive anions such as F- and NO3 - . Finally, we demonstrate hydrazine capture within the second-sphere of metal complexes, followed by unique activation pathways to generate hydrazido and diazene ligands on Zn and Fe, respectively.
Keywords: Chemoselective Anion Binding; Ditopic Boranes; Hydrazine Functionalization; Lewis Acids; Secondary Coordination Sphere.
© 2023 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Figures




References
-
- a) Borovik AS, Acc. Chem. Res 2005, 38, 54–61; - PubMed
- b) Spatzal T, Perez KA, Einsle O, Howard JB, Rees DC, Science 2014, 345, 1620–1623; - PMC - PubMed
- c) Sippel D, Rohde M, Netzer J, Trncik C, Gies J, Grunau K, Djurdjevic I, Decamps L, Andrade SLA, Einsle O, Science 2018, 359, 1484–1489; - PubMed
- d) Van Stappen C, Deng Y, Liu Y, Heidari H, Wang J-X, Zhou Y, Ledray AP, Lu Y, Chem. Rev 2022, 122, 11974–12045; - PMC - PubMed
- e) Stripp ST, Duffus BR, Fourmond V, Léger C, Leimkühler S, Hirota S, Hu Y, Jasniewski A, Ogata H, Ribbe MW, Chem. Rev 2022, 122, 11900–11973. - PMC - PubMed
-
- a) Dos Santos PC, Igarashi RY, Lee H-I, Hoffman BM, Seefeldt LC, Dean DR, Acc. Chem. Res 2005, 38, 208–214; - PubMed
- b) Zhang X, Houk KN, Acc. Chem. Res 2005, 38, 379–385; - PubMed
- c) Prejanò M, Medina FE, Ramos MJ, Russo N, Fernandes PA, Marino T, ACS Catal 2020, 10, 2872–2881; - PMC - PubMed
- d) Warshel A, Sharma PK, Kato M, Xiang Y, Liu H, Olsson MHM, Chem. Rev 2006, 106, 3210–3235. - PubMed
-
- Drover MW, Chem. Soc. Rev 2022, 51, 1861–1880. - PubMed
-
- Miller AJM, Labinger JA, Bercaw JE, J. Am. Chem. Soc 2008, 130, 11874–11875. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

