PRMT5 is a therapeutic target in choroidal neovascularization
- PMID: 36720900
- PMCID: PMC9889383
- DOI: 10.1038/s41598-023-28215-w
PRMT5 is a therapeutic target in choroidal neovascularization
Abstract
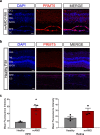
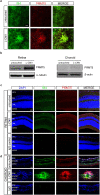
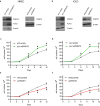
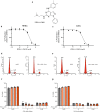
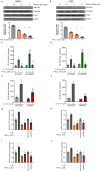
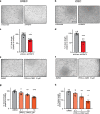
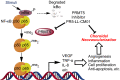
Ocular neovascular diseases including neovascular age-related macular degeneration (nvAMD) are widespread causes of blindness. Patients' non-responsiveness to currently used biologics that target vascular endothelial growth factor (VEGF) poses an unmet need for novel therapies. Here, we identify protein arginine methyltransferase 5 (PRMT5) as a novel therapeutic target for nvAMD. PRMT5 is a well-known epigenetic enzyme. We previously showed that PRMT5 methylates and activates a proangiogenic and proinflammatory transcription factor, the nuclear factor kappa B (NF-κB), which has a master role in tumor progression, notably in pancreatic ductal adenocarcinoma and colorectal cancer. We identified a potent and specific small molecule inhibitor of PRMT5, PR5-LL-CM01, that dampens the methylation and activation of NF-κB. Here for the first time, we assessed the antiangiogenic activity of PR5-LL-CM01 in ocular cells. Immunostaining of human nvAMD sections revealed that PRMT5 is highly expressed in the retinal pigment epithelium (RPE)/choroid where neovascularization occurs, while mouse eyes with laser induced choroidal neovascularization (L-CNV) showed PRMT5 is overexpressed in the retinal ganglion cell layer and in the RPE/choroid. Importantly, inhibition of PRMT5 by PR5-LL-CM01 or shRNA knockdown of PRMT5 in human retinal endothelial cells (HRECs) and induced pluripotent stem cell (iPSC)-derived choroidal endothelial cells (iCEC2) reduced NF-κB activity and the expression of its target genes, such as tumor necrosis factor α (TNF-α) and VEGF-A. In addition to inhibiting angiogenic properties of proliferation and tube formation, PR5-LL-CM01 blocked cell cycle progression at G1/S-phase in a dose-dependent manner in these cells. Thus, we provide the first evidence that inhibition of PRMT5 impedes angiogenesis in ocular endothelial cells, suggesting PRMT5 as a potential therapeutic target to ameliorate ocular neovascularization.
© 2023. The Author(s).
Conflict of interest statement
A.Mu., M.M., K.S., A.Mo., M.S., T.L., and T.W.C. are named inventors on U.S. Patent Application Number: 18/061,219 related to this work. PR5-LL-CM01 is protected by US Patent Award to T.L. and L.P.. T.L. is the founder of EQon Pharmaceuticals, LLC, a company that owns the licensed patent rights for PR5-LL-CM01 from Indiana University. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Adapting AlphaLISA high throughput screen to discover a novel small-molecule inhibitor targeting protein arginine methyltransferase 5 in pancreatic and colorectal cancers.Oncotarget. 2017 Jun 20;8(25):39963-39977. doi: 10.18632/oncotarget.18102. Oncotarget. 2017. PMID: 28591716 Free PMC article.
-
Inhibition of APE1/Ref-1 redox activity rescues human retinal pigment epithelial cells from oxidative stress and reduces choroidal neovascularization.Redox Biol. 2014 Feb 21;2:485-94. doi: 10.1016/j.redox.2014.01.023. eCollection 2014. Redox Biol. 2014. PMID: 24624338 Free PMC article.
-
Thy-1 Regulates VEGF-Mediated Choroidal Endothelial Cell Activation and Migration: Implications in Neovascular Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2016 Oct 1;57(13):5525-5534. doi: 10.1167/iovs.16-19691. Invest Ophthalmol Vis Sci. 2016. PMID: 27768790 Free PMC article.
-
TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation.Mol Vis. 2016 Feb 3;22:116-28. eCollection 2016. Mol Vis. 2016. PMID: 26900328 Free PMC article.
-
Retinal Inhibition of CCR3 Induces Retinal Cell Death in a Murine Model of Choroidal Neovascularization.PLoS One. 2016 Jun 16;11(6):e0157748. doi: 10.1371/journal.pone.0157748. eCollection 2016. PLoS One. 2016. PMID: 27309355 Free PMC article.
Cited by
-
Oridonin Preserves Retinal Pigmented Epithelial Cell Tight Junctions and Ameliorates Choroidal Neovascularization.Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):56. doi: 10.1167/iovs.66.2.56. Invest Ophthalmol Vis Sci. 2025. PMID: 39982392 Free PMC article.
-
Methylation in cornea and corneal diseases: a systematic review.Cell Death Discov. 2024 Apr 8;10(1):169. doi: 10.1038/s41420-024-01935-2. Cell Death Discov. 2024. PMID: 38589350 Free PMC article. Review.
-
Semaphorin 6A phase separation sustains a histone lactylation-dependent lactate buildup in pathological angiogenesis.Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2423677122. doi: 10.1073/pnas.2423677122. Epub 2025 Apr 17. Proc Natl Acad Sci U S A. 2025. PMID: 40244673
-
Recent advances in engineered exosome-based therapies for ocular vascular disease.J Nanobiotechnology. 2025 Jul 19;23(1):526. doi: 10.1186/s12951-025-03589-3. J Nanobiotechnology. 2025. PMID: 40684186 Free PMC article. Review.
-
Unraveling the Molecular Mechanisms of SIRT7 in Angiogenesis: Insights from Substrate Clues.Int J Mol Sci. 2024 Oct 28;25(21):11578. doi: 10.3390/ijms252111578. Int J Mol Sci. 2024. PMID: 39519130 Free PMC article. Review.
References
-
- Motolani A, Martin M, Sun M, Lu T. NF-κB and cancer therapy drugs. In: Kenakin T, editor. Comprehensive Pharmacology. Elsevier; 2022. pp. 351–363.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

