Notoginsenoside R1 protects against myocardial ischemia/reperfusion injury in mice via suppressing TAK1-JNK/p38 signaling
- PMID: 36721009
- PMCID: PMC10310839
- DOI: 10.1038/s41401-023-01057-y
Notoginsenoside R1 protects against myocardial ischemia/reperfusion injury in mice via suppressing TAK1-JNK/p38 signaling
Abstract
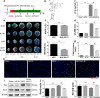
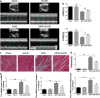
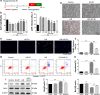
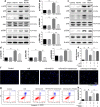
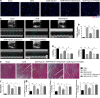
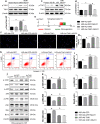
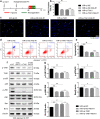
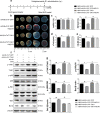
Previous studies show that notoginsenoside R1 (NG-R1), a novel saponin isolated from Panax notoginseng, protects kidney, intestine, lung, brain and heart from ischemia-reperfusion injury. In this study we investigated the cardioprotective mechanisms of NG-R1 in myocardial ischemia/reperfusion (MI/R) injury in vivo and in vitro. MI/R injury was induced in mice by occluding the left anterior descending coronary artery for 30 min followed by 4 h reperfusion. The mice were treated with NG-R1 (25 mg/kg, i.p.) every 2 h for 3 times starting 30 min prior to ischemic surgery. We showed that NG-R1 administration significantly decreased the myocardial infarction area, alleviated myocardial cell damage and improved cardiac function in MI/R mice. In murine neonatal cardiomyocytes (CMs) subjected to hypoxia/reoxygenation (H/R) in vitro, pretreatment with NG-R1 (25 μM) significantly inhibited apoptosis. We revealed that NG-R1 suppressed the phosphorylation of transforming growth factor β-activated protein kinase 1 (TAK1), JNK and p38 in vivo and in vitro. Pretreatment with JNK agonist anisomycin or p38 agonist P79350 partially abolished the protective effects of NG-R1 in vivo and in vitro. Knockdown of TAK1 greatly ameliorated H/R-induced apoptosis of CMs, and NG-R1 pretreatment did not provide further protection in TAK1-silenced CMs under H/R injury. Overexpression of TAK1 abolished the anti-apoptotic effect of NG-R1 and diminished the inhibition of NG-R1 on JNK/p38 signaling in MI/R mice as well as in H/R-treated CMs. Collectively, NG-R1 alleviates MI/R injury by suppressing the activity of TAK1, subsequently inhibiting JNK/p38 signaling and attenuating cardiomyocyte apoptosis.
Keywords: JNK; TAK1; apoptosis; myocardial ischemia/reperfusion injury; notoginsenoside R1; p38.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

