Can Apple and Google continue as health app gatekeepers as well as distributors and developers?
- PMID: 36721023
- PMCID: PMC9889316
- DOI: 10.1038/s41746-023-00754-6
Can Apple and Google continue as health app gatekeepers as well as distributors and developers?
Abstract
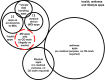
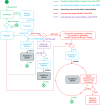
Mobile apps are the primary means by which consumers access digital health and wellness software, with delivery dominated by the 'Apple App Store' and the 'Google Play Store'. Through these virtual storefronts Apple and Google act as the distributor (and sometimes, importer) of many thousands of health and wellness apps into the EU, some of which have a medical purpose. As a result of changes to EU law which came into effect in May 2021, they must now ensure that apps are compliant with medical devices regulation and to inform authorities of serious incidents arising from their use. The extent to which these new rules are being complied with in practice is uneven, and in some areas unclear. In light of EU legislation related to competition, which came into effect in November 2022, it is also unclear how conflicts of interest can be managed between Apple and Google's roles as gateway duopoly importers and distributors whilst also developers of their own competitive health products. Finally, with the proposed European health data space regulation, wellness apps will be voluntarily registered and labelled in a fashion more like medical devices than consumer software. We explore the implications of these new regulations and propose future models that could resolve the apparent conflicts. All stakeholders would benefit from improved app store models to sustainably evolve safer, better, and fairer provision of digital health applications in the EU. As EU legislation comes into force it could serve as a template for other regions globally.
© 2023. The Author(s).
Conflict of interest statement
Author O.S. declares no Competing Financial or Non-Financial Interests. Author S.G. declares no Non-Financial Interests but the following Competing Financial Interests: he has or has had consulting relationships with Una Health GmbH, Lindus Health Ltd.; FLO Ltd, and Thymia Ltd., Ada Health GmbH and holds share options in Ada Health GmbH; Author E.V. declares no Non-Financial Interests and the following Competing Financial Interests: he is a partner of Axon Lawyers. Author H.H. declares no Non-Financial Interests and the following Competing Financial Interests: he is the owner and managing director of Hardian Ltd. Author T.M. declares no Competing Financial Interests and declares the following Non-Financial Interest: he is an unpaid advisory board member of Pumpinheart Ltd.; previously a senior medical officer in medical devices at the Health Products Regulatory Authority, Ireland; previous co-chair of the Clinical Investigation and Evaluation Working Group of the European Commission.
Figures

References
-
- Number of apps available in leading app stores as of 3rd quarter 2022. https://www.statista.com/statistics/276623/number-of-apps-available-in-l... (2022).
-
- IQVIA INSTITUTE. Digital Health Trends 2021 INNOVATION, EVIDENCE, REGULATION, AND ADOPTION. https://www.mobihealthnews.com/news/digital-health-apps-balloon-more-350... (2021).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

