Reprogramming the tumor microenvironment with biotechnology
- PMID: 36721212
- PMCID: PMC9890796
- DOI: 10.1186/s40824-023-00343-4
Reprogramming the tumor microenvironment with biotechnology
Abstract
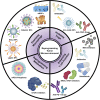
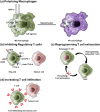
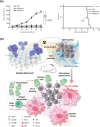
The tumor microenvironment (TME) is a unique environment that is developed by the tumor and controlled by tumor-induced interactions with host cells during tumor progression. The TME includes immune cells, which can be classified into two types: tumor- antagonizing and tumor-promoting immune cells. Increasing the tumor treatment responses is associated with the tumor immune microenvironment. Targeting the TME has become a popular topic in research, which includes polarizing macrophage phenotype 2 into macrophage phenotype 1 using Toll-like receptor agonists with cytokines, anti-CD47, and anti-SIPRα. Moreover, inhibiting regulatory T cells through blockades and depletion restricts immunosuppressive cells in the TME. Reprogramming T cell infiltration and T cell exhaustion improves tumor infiltrating lymphocytes, such as CD8+ or CD4+ T cells. Targeting metabolic pathways, including glucose, lipid, and amino acid metabolisms, can suppress tumor growth by restricting the absorption of nutrients and adenosine triphosphate energy into tumor cells. In conclusion, these TME reprogramming strategies exhibit more effective responses using combination treatments, biomaterials, and nanoparticles. This review highlights how biomaterials and immunotherapy can reprogram TME and improve the immune activity.
Keywords: Biomaterials; Combination treatment; Nanoparticle; Reprogramming; Tumor microenvironment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing of interest or personal relationships that could have seemed to affect the work reported in this paper.
Figures


References
-
- Cao Y, DePinho RA, Ernst M, Vousden K. Cancer research: past, present and future. Nat Rev Cancer. 2011;11:749–754. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

