In vitro toxicological assessment of PhSeZnCl in human liver cells
- PMID: 36721677
- PMCID: PMC9839901
- DOI: 10.1007/s43188-022-00148-y
In vitro toxicological assessment of PhSeZnCl in human liver cells
Abstract
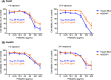
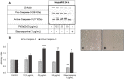
Phenylselenenylzinc chloride (PhSeZnCl) is an air-stable selenolate, easily synthesizable through oxidative insertion of elemental zinc into the Se-halogen bond of the commercially available phenylselenyl chloride. PhSeZnCl was shown to possess a marked GPx-like activity both in NMR and in vitro tests, and to effectively react with cellular thiols, and was supposed for a potential use in the chemotherapy of drug-resistant cancers. However, activity of PhSeZnCl in hepatic cells has never been tested before now. In this in vitro approach, we evaluated the cytotoxic, genotoxic, and apoptotic activities, as well as the effects on cell cycle of PhSeZnCl in two preclinical hepatic models, namely HepG2 and HepaRG cells. Results showed that cell viability of HepG2 and HepaRG cells decreased in a dose-dependent manner, with a more marked effect in HepG2 tumour cells. Moreover, treatment with 50 µg/mL PhSeZnCl caused an increase of primary DNA damage (4 h) and a statistically significant increase of HepG2 cells arrested in G2/M phase. In addition, it altered mitochondrial membrane potential and induced chromosomal DNA fragmentation (24 h). In HepaRG cells, PhSeZnCl was able to determine a cell cycle-independent induction of apoptosis. Particularly, 50 µg/mL induced mitochondrial membrane depolarization after 24 h and apoptosis after 4 h treatment. Futhermore, all PhSeZnCl concentrations tested determined a significant increase of apoptotic cells after 24 h. Apoptosis was also highlighted by the detection of active Caspase-3 by Western Blot analysis after 24 h exposure. In conclusion, this first toxicological assessment provides new insights into the biological activity of PhSeZnCl in preclinical hepatic models that will be useful in future safety assessment investigation of this compound as a potential pharmaceutical.
Supplementary information: The online version contains supplementary material available at 10.1007/s43188-022-00148-y.
Keywords: Apoptosis; Cell cycle; Comet assay; HepG2; HepaRG; Phenylselenenylzinc chloride.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors declare that there is no conflict of interest regarding the publication of this paper.
Figures
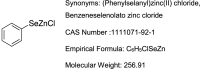

References
LinkOut - more resources
Full Text Sources
Research Materials

