Spatio-temporal remodelling of the composition and architecture of the human ovarian cortical extracellular matrix during in vitro culture
- PMID: 36721914
- PMCID: PMC9977129
- DOI: 10.1093/humrep/dead008
Spatio-temporal remodelling of the composition and architecture of the human ovarian cortical extracellular matrix during in vitro culture
Abstract
Study question: How does in vitro culture alter the human ovarian cortical extracellular matrix (ECM) network structure?
Summary answer: The ECM composition and architecture vary in the different layers of the ovarian cortex and are remodelled during in vitro culture.
What is known already: The ovarian ECM is the scaffold within which follicles and stromal cells are organized. Its composition and structural properties constantly evolve to accommodate follicle development and expansion. Tissue preparation for culture of primordial follicles within the native ECM involves mechanical loosening; this induces undefined modifications in the ECM network and alters cell-cell contact, leading to spontaneous follicle activation.
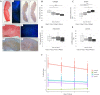
Study design, size, duration: Fresh ovarian cortical biopsies were obtained from six women aged 28-38 years (mean ± SD: 32.7 ± 4.1 years) at elective caesarean section. Biopsies were cut into fragments of ∼4 × 1 × 1 mm and cultured for 0, 2, 4, or 6 days (D).
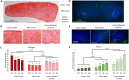
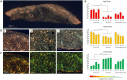
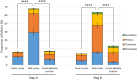
Participants/materials, setting, methods: Primordial follicle activation, stromal cell density, and ECM-related protein (collagen, elastin, fibronectin, laminin) positive area in the entire cortex were quantified at each time point using histological and immunohistological analysis. Collagen and elastin content, collagen fibre characteristics, and follicle distribution within the tissue were further quantified within each layer of the human ovarian cortex, namely the outer cortex, the mid-cortex, and the cortex-medulla junction regions.
Main results and the role of chance: Primordial follicle activation occurred concomitantly with a loosening of the ovarian cortex during culture, characterized by an early decrease in stromal cell density from 3.6 ± 0.2 × 106 at day 0 (D0) to 2.8 ± 0.1 × 106 cells/mm3 at D2 (P = 0.033) and a dynamic remodelling of the ECM. Notably, collagen content gradually fell from 55.5 ± 1.7% positive area at D0 to 42.3 ± 1.1% at D6 (P = 0.001), while elastin increased from 1.1 ± 0.2% at D0 to 1.9 ± 0.1% at D6 (P = 0.001). Fibronectin and laminin content remained stable. Moreover, collagen and elastin distribution were uneven throughout the cortex and during culture. Analysis at the sub-region level showed that collagen deposition was maximal in the outer cortex and the lowest in the mid-cortex (69.4 ± 1.2% versus 53.8 ± 0.8% positive area, respectively, P < 0.0001), and cortical collagen staining overall decreased from D0 to D2 (65.2 ± 2.4% versus 60.6 ± 1.8%, P = 0.033) then stabilized. Elastin showed the converse distribution, being most concentrated at the cortex-medulla junction (3.7 ± 0.6% versus 0.9 ± 0.2% in the outer cortex, P < 0.0001), and cortical elastin peaked at D6 compared to D0 (3.1 ± 0.5% versus 1.3 ± 0.2%, P < 0.0001). This was corroborated by a specific signature of the collagen fibre type across the cortex, indicating a distinct phenotype of the ovarian cortical ECM depending on region and culture period that might be responsible for the spatio-temporal and developmental pattern of follicular distribution observed within the cortex.
Large scale data: N/A.
Limitations, reasons for caution: Ovarian cortical biopsies were obtained from women undergoing caesarean sections. As such, the data obtained may not accurately reflect the ECM distribution and structure of non-pregnant women.
Wider implications of the findings: Clarifying the composition and architecture signature of the human ovarian cortical ECM provides a foundation for further exploration of ovarian microenvironments. It is also critical for understanding the ECM-follicle interactions regulating follicle quiescence and awakening, leading to improvements in both in vitro activation and in vitro growth techniques.
Study funding/competing interest(s): Medical Research Council grant MR/R003246/1 and Wellcome Trust Collaborative Award in Science: 215625/Z/19/Z. The authors have no conflicts to declare.
Trial registration number: N/A.
Keywords: extracellular matrix; fertility preservation; mechanobiology; ovary; primordial follicle activation; tissue stiffness.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures



References
-
- Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z.. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod 1999;14:1299–1301. - PubMed
-
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S.. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013;154:1047–1059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

