Purine synthesis suppression reduces the development and progression of pulmonary hypertension in rodent models
- PMID: 36721994
- PMCID: PMC10319969
- DOI: 10.1093/eurheartj/ehad044
Purine synthesis suppression reduces the development and progression of pulmonary hypertension in rodent models
Abstract
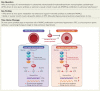
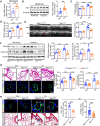
Aims: Proliferation of vascular smooth muscle cells (VSMCs) is a hallmark of pulmonary hypertension (PH). Proliferative cells utilize purine bases from the de novo purine synthesis (DNPS) pathways for nucleotide synthesis; however, it is unclear whether DNPS plays a critical role in VSMC proliferation during development of PH. The last two steps of DNPS are catalysed by the enzyme 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/inosine monophosphate cyclohydrolase (ATIC). This study investigated whether ATIC-driven DNPS affects the proliferation of pulmonary artery smooth muscle cells (PASMCs) and the development of PH.
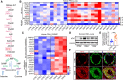
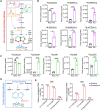
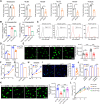
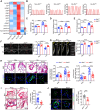
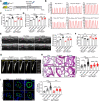
Methods and results: Metabolites of DNPS in proliferative PASMCs were measured by liquid chromatography-tandem mass spectrometry. ATIC expression was assessed in platelet-derived growth factor-treated PASMCs and in the lungs of PH rodents and patients with pulmonary arterial hypertension. Mice with global and VSMC-specific knockout of Atic were utilized to investigate the role of ATIC in both hypoxia- and lung interleukin-6/hypoxia-induced murine PH. ATIC-mediated DNPS at the mRNA, protein, and enzymatic activity levels were increased in platelet-derived growth factor-treated PASMCs or PASMCs from PH rodents and patients with pulmonary arterial hypertension. In cultured PASMCs, ATIC knockdown decreased DNPS and nucleic acid DNA/RNA synthesis, and reduced cell proliferation. Global or VSMC-specific knockout of Atic attenuated vascular remodelling and inhibited the development and progression of both hypoxia- and lung IL-6/hypoxia-induced PH in mice.
Conclusion: Targeting ATIC-mediated DNPS compromises the availability of purine nucleotides for incorporation into DNA/RNA, reducing PASMC proliferation and pulmonary vascular remodelling and ameliorating the development and progression of PH.
Keywords: De novo purine synthesis; ATIC; Pulmonary hypertension; Vascular smooth muscle cells.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement All authors declare no conflict of interest for this contribution.
Figures

Comment in
-
De novo purine synthesis: a new target in pulmonary arterial hypertension?Eur Heart J. 2023 Apr 7;44(14):1280-1282. doi: 10.1093/eurheartj/ehad078. Eur Heart J. 2023. PMID: 36805649 No abstract available.
-
Focus issue on vascular biology and medicine spanning from management of stroke to new therapeutic targets in aortic dissection and pulmonary hypertension.Eur Heart J. 2023 Apr 7;44(14):1193-1196. doi: 10.1093/eurheartj/ehad198. Eur Heart J. 2023. PMID: 37024112 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

