Molecular basis of the different effects of procainamide and N-acetylprocainamide on the maximum upstroke velocity and half-decay time of the cardiac action potential in guinea pig papillary muscle
- PMID: 36722655
- PMCID: PMC9883003
- DOI: 10.1590/1414-431X2023e12073
Molecular basis of the different effects of procainamide and N-acetylprocainamide on the maximum upstroke velocity and half-decay time of the cardiac action potential in guinea pig papillary muscle
Abstract
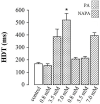
Procainamide (PA) and its in vivo metabolite, N-acetylprocainamide (NAPA), display some pharmacological differences. Although it is agreed that PA is a class IA antiarrhythmic, it has been reported that NAPA is a pure class III antiarrhythmic that affects only the repolarizing phase of the cardiac action potential. This last concept, observed exclusively in dogs, gained wide acceptance, appearing in classic pharmacology textbooks. However, evidence in species such as mice and rats indicates that NAPA can affect cardiac Na+ channels, which is unexpected for a pure class III antiarrhythmic drug. To further clarify this issue, the effects of PA (used as a reference drug) and NAPA on the maximum upstroke velocity (Vmax) and half-decay time (HDT) of the cardiac action potential were examined in the isolated right papillaris magnus of the guinea pig heart. Both PA and NAPA affected Vmax at lower concentrations than required to affect HDT, and NAPA had weaker effects on both variables. Thus, NAPA displayed typical class IA antiarrhythmic behavior. Therefore, the concept that NAPA is a pure class III antiarrhythmic drug is more species-dependent than previously envisioned. In addition, we demonstrated that the differential pharmacology of PA and NAPA is explainable, in molecular terms, by steric hindrance of the effects of NAPA and the greater number of potent aromatic-aromatic and cation π interactions with Na+ or K+ cardiac channels for PA.
Figures



References
-
- Armstrong EJ, Clapham DE. In: Pharmacology of cardiac rhythm. Golan DE, Tashjian AH Jr, Armstrong EJ, Armstrong AW, editors. Philadelphia: Wolters Kluwer; 2017. pp. 433–453.
-
- Knollmann BC, Roden DM. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th ed. Brunton LL, Hilal-Dandan R, Knollman B, editors. New York: McGraw-Hill; 2018. Antiarrhythmic drugs; pp. 547–572.
-
- Bagwell EE, Walle T, Drayer DE, Reidensberg MM, Pruett JK. Correlation of the electrophysiological and antiarrhythmic properties of the N-acetyl metabolite of procainamide with plasma and tissue drug concentrations in the dog. J Pharmacol Exp Ther. 1976;197:38–48. - PubMed
-
- Dangman KH, Hoffman BF. In vivo and in vitro antiarrhythmic and arrhythmogenic effects of N-acetylprocainamide. J Pharmacol Exp Ther. 1981;217:851–862. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

