Mechanism of the Early Catalytic Events in the Collagenolysis by Matrix Metalloproteinase-1
- PMID: 36723036
- PMCID: PMC12330993
- DOI: 10.1002/cphc.202200943
Mechanism of the Early Catalytic Events in the Collagenolysis by Matrix Metalloproteinase-1
Abstract
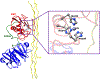
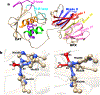
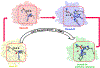
The front cover artwork is provided by Dr. Karabencheva-Christova's group at Michigan Technological University. The images show the initially formed and the catalytically productive conformations of MMP-1 complex with the Triple Helical Peptide (THP), the free energy profile connecting them as well as the coordination geometry of the catalytic zinc (II). The background shows the collagen macromolecule. Read the full text of the Research Article at 10.1002/cphc.202200649.
© 2023 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors declare no competing financial interest.
Figures
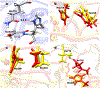



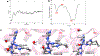

References
-
- Streuli C. Extracellular Matrix Remodelling and Cellular Differentiation. Curr. Opin. Cell Biol 1999, 11 (5), 634–640. - PubMed
-
- Weaver VM; Roskelley CD Extracellular Matrix: The Central Regulator of Cell and Tissue Homeostasis. Trends Cell Biol. 1997, 7 (1), 40–42. - PubMed
-
- Rosenberg GA Matrix Metalloproteinases and Their Multiple Roles in Neurodegenerative Diseases. Lancet Neurol. 2009, 8 (2), 205–216. - PubMed
-
- Gupta SP; Patil VM Specificity of Binding with Matrix Metalloproteinases. Exp. Suppl. 2012 2012, 103, 35–56. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

