Magnesium Homeostasis: Lessons from Human Genetics
- PMID: 36723340
- PMCID: PMC10356123
- DOI: 10.2215/CJN.0000000000000103
Magnesium Homeostasis: Lessons from Human Genetics
Abstract
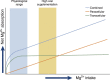
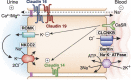
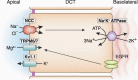
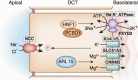
Mg 2+ , the fourth most abundant cation in the body, serves as a cofactor for about 600 cellular enzymes. One third of ingested Mg 2+ is absorbed from the gut through a saturable transcellular process and a concentration-dependent paracellular process. Absorbed Mg 2+ is excreted by the kidney and maintains serum Mg 2+ within a narrow range of 0.7-1.25 mmol/L. The reabsorption of Mg 2+ by the nephron is characterized by paracellular transport in the proximal tubule and thick ascending limb. The nature of the transport pathways in the gut epithelia and thick ascending limb has emerged from an understanding of the molecular mechanisms responsible for rare monogenetic disorders presenting with clinical hypomagnesemia. These human disorders due to loss-of-function mutations, in concert with mouse models, have led to a deeper understanding of Mg 2+ transport in the gut and renal tubule. This review focuses on the nature of the transporters and channels revealed by human and mouse genetics and how they are integrated into an understanding of human Mg 2+ physiology.
Copyright © 2023 by the American Society of Nephrology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

