Safety and efficacy of long-acting injectable cabotegravir as preexposure prophylaxis to prevent HIV acquisition
- PMID: 36723489
- PMCID: PMC10090368
- DOI: 10.1097/QAD.0000000000003494
Safety and efficacy of long-acting injectable cabotegravir as preexposure prophylaxis to prevent HIV acquisition
Abstract
Objective: HIV remains a significant burden, despite expanding HIV prevention tools. Long-acting injectable cabotegravir (CAB-LA) is a new preexposure prophylaxis (PrEP) product. We reviewed existing evidence to determine the efficacy and safety of CAB-LA as PrEP to inform global guidelines.
Design: Systematic review and meta-analysis.
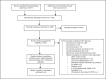
Methods: We systematically reviewed electronic databases and conference abstracts for citations on CAB-LA from January 2010 to September 2021. Outcomes included HIV infection, adverse events, drug resistance, pregnancy-related adverse events, and sexual behavior. We calculated pooled effect estimates using random-effects meta-analysis and summarized other results narratively.
Results: We identified 12 articles/abstracts representing four multisite randomized controlled trials. Study populations included cisgender men, cisgender women, and transgender women. The pooled relative risk of HIV acquisition comparing CAB-LA to oral PrEP within efficacy studies was 0.21 (95% confidence interval: 0.07-0.61), resulting in a 79% reduction in HIV risk. Rates of adverse events were similar across study groups. Of 19 HIV infections among those randomized to CAB-LA with results available, seven had integrase strand transfer inhibitor (INSTI) resistance. Data on pregnancy-related adverse events were sparse. No studies reported on sexual behavior.
Conclusions: CAB-LA is highly efficacious for HIV prevention with few safety concerns. CAB-LA may lead to an increased risk of INSTI resistance among those who have acute HIV infection at initiation or become infected while taking CAB-LA. However, results are limited to controlled studies; more research is needed on real-world implementation. Additional data are needed on the safety of CAB-LA during pregnancy (for mothers and infants) and among populations not included in the trials.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- UNAIDS. Global HIV & AIDS statistics — fact sheet. Geneva: UNAIDS; 2022. Available at: https://www.unaids.org/en/resources/fact-sheet.
-
- World Health Organization, World Health Organization. Guideline on when to start antiretroviral therapy and on preexposure prophylaxis for HIV. 2015. - PubMed
-
- World Health Organization. What's the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV for men who have sex with men: update to WHO's recommendation on oral PrEP. Geneva: WHO; 2019.
-
- World Health Organization. Guidelines: updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. Geneva: WHO; 2021. - PubMed
-
- Celum C, Baeten J. PrEP for HIV prevention: evidence, global scale-up, and emerging options. Cell Host Microbe 2020; 27:502–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous